Int J Stem Cells.
2021 Nov;14(4):455-464. 10.15283/ijsc21005.
Irisin Enhances Angiogenesis of Mesenchymal Stem Cells to Promote Cardiac Function in Myocardial Infarction via PI3k/Akt Activation
- Affiliations
-
- 1Department of Cardiology, Guizhou Provincial People’s Hospital, Guiyang, China
- 2Department of Cardiology, Guizhou University People’s Hospital, Guiyang, China
- 3Qingdao Municipal Hospital (Group), Qingdao, China
- 4Department of Cardiology, Xixiu District People’s Hospital, Anshun, China
- KMID: 2522563
- DOI: http://doi.org/10.15283/ijsc21005
Abstract
- Background and Objectives
With the growing incidence of acute myocardial infarction (MI), angiogenesis is vital for cardiac function post-MI. The role of bone marrow mesenchymal stem cells (BMSCs) in angiogenesis has been previously confirmed. Irisin is considered a potential vector for angiogenesis. The objective of the present study was to investigate the potential role of irisin in the angiogenesis of BMSCs.
Methods and Results
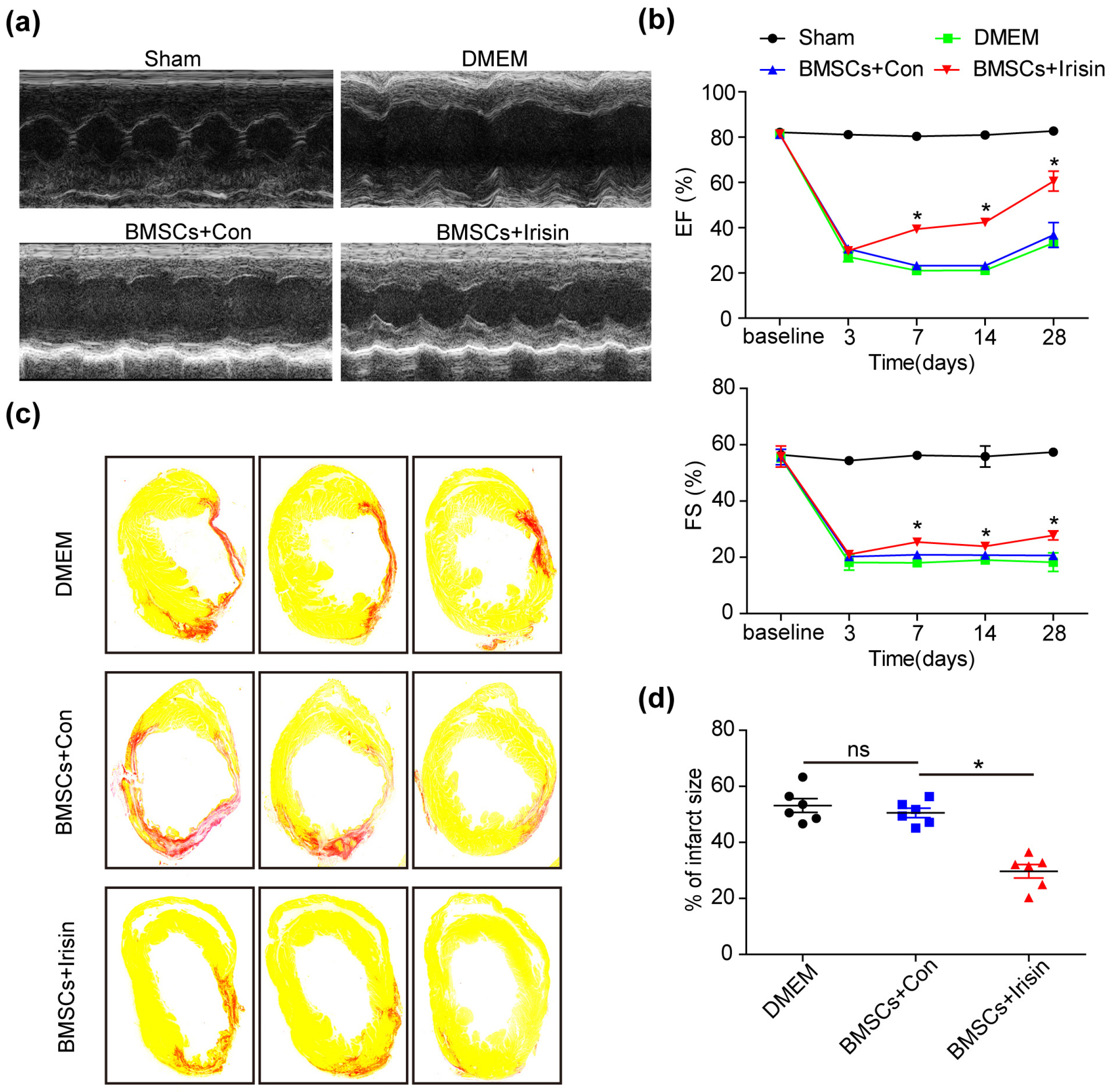
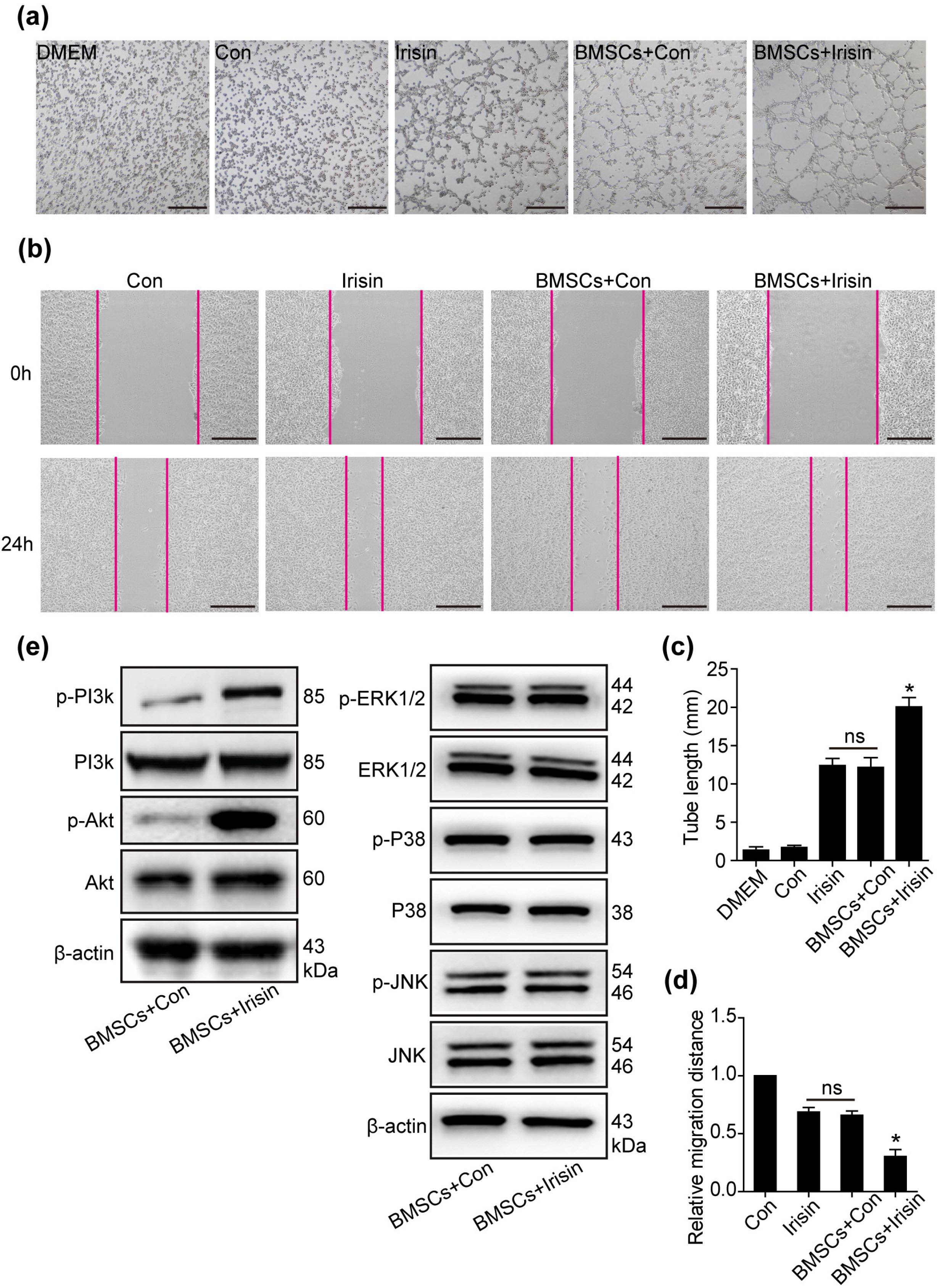
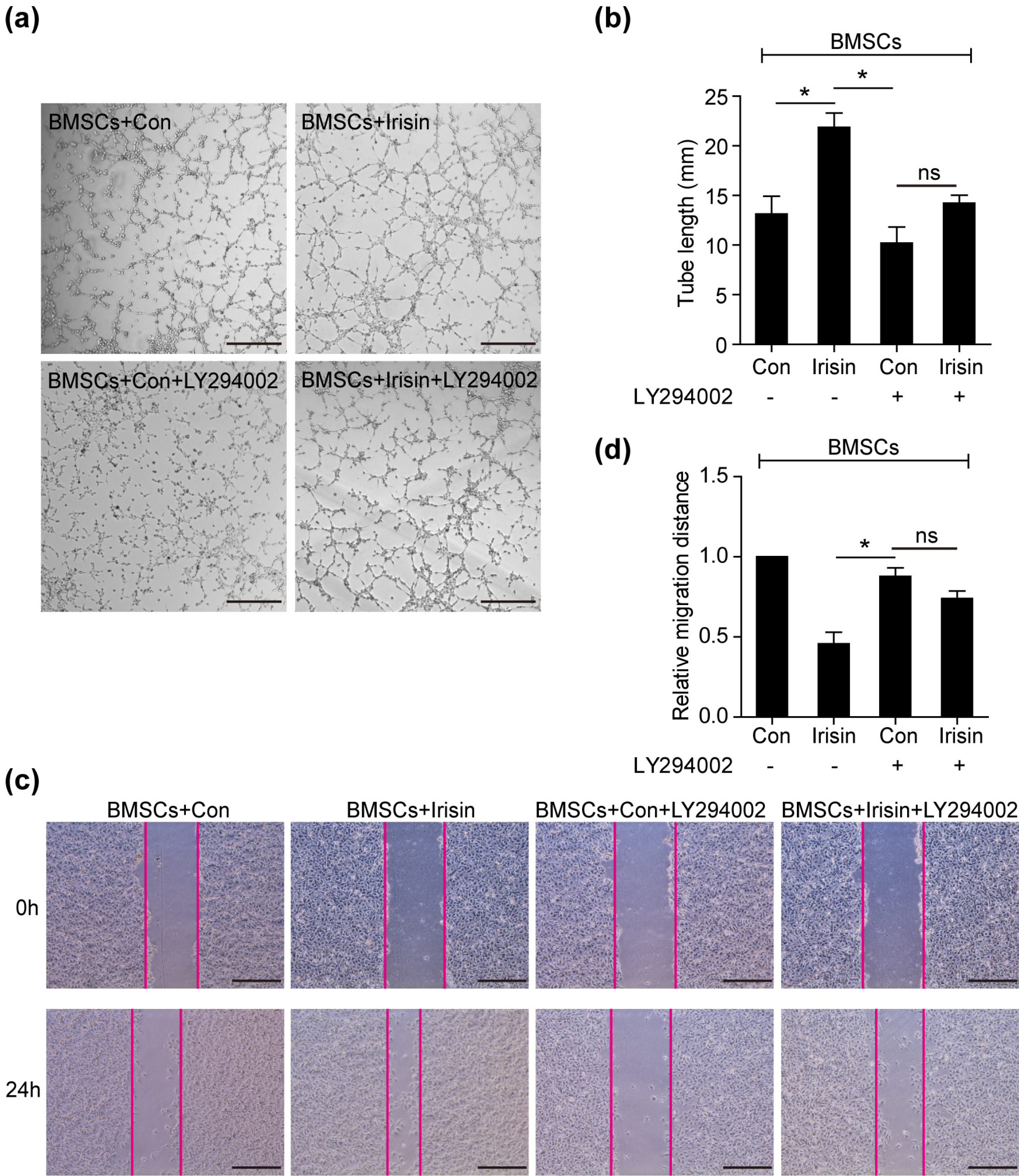
In vivo, irisin-treated BMSCs (BMSCs+irisin) were transplanted into an MI mouse model. On day 28 post-MI, blood vessel markers were detected, and cardiac function and infarct areas of mice were evaluated. In vitro, paracrine effects were assessed by examining tube formation in human umbilical vein endothelial cells (HUVECs) co-cultured with the BMSCs+irisin supernatant. The scratch wound-healing assay was performed to evaluate HUVEC migration. Western blotting was performed to determine PI3k/Akt pathway activation in the BMSCs+irisin group. Transplantation of BMSCs+irisin promoted greater angiogenesis, resulting in better cardiac function in the MI mouse model than in controls. In the BMSC+irisin group, HUVECs demonstrated enhanced tube formation and migration. Activation of the PI3k/Akt pathway was found to be involved in mediating the role of irisin in the angiogenesis of BMSCs.
Conclusions
In cardiovascular diseases such as MI, irisin administration can enhance angiogenesis of BMSCs and pro-mote cardiac function via the PI3k/Akt pathway, optimizing the therapeutic effect based on BMSCs transplantation.
Keyword
Figure
Reference
-
References
1. Bubb KJ, Aubdool AA, Moyes AJ, Lewis S, Drayton JP, Tang O, Mehta V, Zachary IC, Abraham DJ, Tsui J, Hobbs AJ. 2019; Endothelial C-type natriuretic peptide is a critical regulator of angiogenesis and vascular remodeling. Circulation. 139:1612–1628. DOI: 10.1161/CIRCULATIONAHA.118.036344. PMID: 30586761. PMCID: PMC6438487.
Article2. Oka T, Akazawa H, Naito AT, Komuro I. 2014; Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 114:565–571. DOI: 10.1161/CIRCRESAHA.114.300507. PMID: 24481846.3. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. 2012; A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 481:463–468. DOI: 10.1038/nature10777. PMID: 22237023. PMCID: PMC3522098.
Article4. Tachibana A, Santoso MR, Mahmoudi M, Shukla P, Wang L, Bennett M, Goldstone AB, Wang M, Fukushi M, Ebert AD, Woo YJ, Rulifson E, Yang PC. 2017; Paracrine effects of the pluripotent stem cell-derived cardiac myocytes salvage the injured myocardium. Circ Res. 121:e22–e36. DOI: 10.1161/CIRCRESAHA.117.310803. PMID: 28743804. PMCID: PMC5783162.
Article5. Wang H, Zhao YT, Zhang S, Dubielecka PM, Du J, Yano N, Chin YE, Zhuang S, Qin G, Zhao TC. 2017; Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J Cell Physiol. 232:3775–3785. DOI: 10.1002/jcp.25857. PMID: 28181692. PMCID: PMC5550372.
Article6. Zhang X, Hu C, Kong CY, Song P, Wu HM, Xu SC, Yuan YP, Deng W, Ma ZG, Tang QZ. 2020; FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 27:540–555. DOI: 10.1038/s41418-019-0372-z. PMID: 31209361. PMCID: PMC7206111.
Article7. Yu Q, Kou W, Xu X, Zhou S, Luan P, Xu X, Li H, Zhuang J, Wang J, Zhao Y, Xu Y, Peng W. 2019; FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clin Sci (Lond). 133:611–627. DOI: 10.1042/CS20190016. PMID: 30782608.
Article8. Li RL, Wu SS, Wu Y, Wang XX, Chen HY, Xin JJ, Li H, Lan J, Xue KY, Li X, Zhuo CL, Cai YY, He JH, Zhang HY, Tang CS, Wang W, Jiang W. 2018; Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J Mol Cell Cardiol. 121:242–255. DOI: 10.1016/j.yjmcc.2018.07.250. PMID: 30053525.
Article9. Wu F, Song H, Zhang Y, Zhang Y, Mu Q, Jiang M, Wang F, Zhang W, Li L, Li H, Wang Y, Zhang M, Li S, Yang L, Meng Y, Tang D. 2015; Irisin induces angiogenesis in human umbilical vein endothelial cells in vitro and in zebrafish embryos in vivo via activation of the ERK signaling pathway. PLoS One. 10:e0134662. DOI: 10.1371/journal.pone.0134662. PMID: 26241478. PMCID: PMC4524626.
Article10. Liao Q, Qu S, Tang LX, Li LP, He DF, Zeng CY, Wang WE. 2019; Irisin exerts a therapeutic effect against myocardial infarction via promoting angiogenesis. Acta Pharmacol Sin. 40:1314–1321. DOI: 10.1038/s41401-019-0230-z. PMID: 31061533. PMCID: PMC6786355.
Article11. Yang F, Wu R, Jiang Z, Chen J, Nan J, Su S, Zhang N, Wang C, Zhao J, Ni C, Wang Y, Hu W, Zeng Z, Zhu K, Liu X, Hu X, Zhu W, Yu H, Huang J, Wang J. 2018; Leptin increases mitochondrial OPA1 via GSK3-mediated OMA1 ubiquitination to enhance therapeutic effects of mesenchymal stem cell transplantation. Cell Death Dis. 9:556. DOI: 10.1038/s41419-018-0579-9. PMID: 29748581. PMCID: PMC5945599.
Article12. Zhang N, Ye F, Zhu W, Hu D, Xiao C, Nan J, Su S, Wang Y, Liu M, Gao K, Hu X, Chen J, Yu H, Xie X, Wang J. 2016; Cardiac ankyrin repeat protein attenuates cardiomyocyte apoptosis by upregulation of Bcl-2 expression. Biochim Biophys Acta. 1863:3040–3049. DOI: 10.1016/j.bbamcr.2016.09.024. PMID: 27713078.
Article13. Golpanian S, Wolf A, Hatzistergos KE, Hare JM. 2016; Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol Rev. 96:1127–1168. DOI: 10.1152/physrev.00019.2015. PMID: 27335447. PMCID: PMC6345247.
Article14. Ranganath SH, Levy O, Inamdar MS, Karp JM. 2012; Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 10:244–258. DOI: 10.1016/j.stem.2012.02.005. PMID: 22385653. PMCID: PMC3294273.
Article15. Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, Dilley RJ. 2012; Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 21:2189–2203. DOI: 10.1089/scd.2011.0674. PMID: 22188562. PMCID: PMC3411362.
Article16. Deng J, Zhang N, Wang Y, Yang C, Wang Y, Xin C, Zhao J, Jin Z, Cao F, Zhang Z. 2020; FNDC5/irisin improves the therapeutic efficacy of bone marrow-derived mesenchymal stem cells for myocardial infarction. Stem Cell Res Ther. 11:228. DOI: 10.1186/s13287-020-01746-z. PMID: 32522253. PMCID: PMC7288492.
Article17. Mahajan UB, Chandrayan G, Patil CR, Arya DS, Suchal K, Agrawal Y, Ojha S, Goyal SN. 2018; Eplerenone attenuates myocardial infarction in diabetic rats via modulation of the PI3K-Akt pathway and phosphorylation of GSK-3β. Am J Transl Res. 10:2810–2821. PMID: 30323868. PMCID: PMC6176230.18. Wollert KC, Drexler H. 2010; Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 7:204–215. DOI: 10.1038/nrcardio.2010.1. PMID: 20177405.
Article19. Parekkadan B, Milwid JM. 2010; Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 12:87–117. DOI: 10.1146/annurev-bioeng-070909-105309. PMID: 20415588. PMCID: PMC3759519.
Article20. Park J, Lee JH, Yoon BS, Jun EK, Lee G, Kim IY, You S. 2018; Additive effect of bFGF and selenium on expansion and paracrine action of human amniotic fluid-derived mesenchymal stem cells. Stem Cell Res Ther. 9:293. DOI: 10.1186/s13287-018-1058-z. PMID: 30409167. PMCID: PMC6225588.
Article21. Korta P, Pocheć E, Mazur-Biały A. 2019; Irisin as a multifunctional protein: implications for health and certain diseases. Medicina (Kaunas). 55:485. DOI: 10.3390/medicina55080485. PMID: 31443222. PMCID: PMC6722973.
Article22. Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, Gonçalves RA, Clarke JR, Beckman D, Staniszewski A, Berman H, Guerra LA, Forny-Germano L, Meier S, Wilcock DM, de Souza JM, Alves-Leon S, Prado VF, Prado MAM, Abisambra JF, Tovar-Moll F, Mattos P, Arancio O, Ferreira ST, De Felice FG. 2019; Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat Med. 25:165–175. DOI: 10.1038/s41591-018-0275-4. PMID: 30617325. PMCID: PMC6327967.
Article23. Zhou X, Xu M, Bryant JL, Ma J, Xu X. 2019; Exercise-induced myokine FNDC5/irisin functions in cardiovascular protection and intracerebral retrieval of synaptic plasticity. Cell Biosci. 9:32. DOI: 10.1186/s13578-019-0294-y. PMID: 30984367. PMCID: PMC6446275.
Article24. Tan Y, Ouyang H, Xiao X, Zhong J, Dong M. 2019; Irisin ameliorates septic cardiomyopathy via inhibiting DRP1-related mitochondrial fission and normalizing the JNK-LATS2 signaling pathway. Cell Stress Chaperones. 24:595–608. DOI: 10.1007/s12192-019-00992-2. PMID: 30993599. PMCID: PMC6527615.
Article25. Colaianni G, Mongelli T, Colucci S, Cinti S, Grano M. 2016; Crosstalk between muscle and bone via the muscle-myokine Irisin. Curr Osteoporos Rep. 14:132–137. DOI: 10.1007/s11914-016-0313-4. PMID: 27299471.
Article26. Grygiel-Górniak B, Puszczewicz M. 2017; A review on irisin, a new protagonist that mediates muscle-adipose-bone-neuron connectivity. Eur Rev Med Pharmacol Sci. 21:4687–4693. PMID: 29131244.27. Lee CW, Hsiao WT, Lee OK. 2017; Mesenchymal stromal cell-based therapies reduce obesity and metabolic syndromes induced by a high-fat diet. Transl Res. 182:61–74.e8. DOI: 10.1016/j.trsl.2016.11.003. PMID: 27908750.
Article28. Yao D, Liu NN, Mo BW. 2020; Assessment of proliferation, migration and differentiation potentials of bone marrow mesenchymal stem cells labeling with silica-coated and amine-modified superparamagnetic iron oxide nanoparticles. Cytotechnology. 72:513–525. DOI: 10.1007/s10616-020-00397-5. PMID: 32394163. PMCID: PMC7450019.
Article29. Ullah M, Liu DD, Thakor AS. 2019; Mesenchymal stromal cell homing: mechanisms and strategies for improvement. iScience. 15:421–438. DOI: 10.1016/j.isci.2019.05.004. PMID: 31121468. PMCID: PMC6529790.
Article30. Rabiee F, Lachinani L, Ghaedi S, Nasr-Esfahani MH, Megraw TL, Ghaedi K. 2020; New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 10:51. DOI: 10.1186/s13578-020-00413-3. PMID: 32257109. PMCID: PMC7106581.
Article31. Ersahin T, Tuncbag N, Cetin-Atalay R. 2015; The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 11:1946–1954. DOI: 10.1039/C5MB00101C. PMID: 25924008.
Article32. Lanza Cariccio V, Scionti D, Raffa A, Iori R, Pollastro F, Diomede F, Bramanti P, Trubiani O, Mazzon E. 2018; Treatment of periodontal ligament stem cells with MOR and CBD promotes cell survival and neuronal differentiation via the PI3K/Akt/mTOR pathway. Int J Mol Sci. 19:2341. DOI: 10.3390/ijms19082341. PMID: 30096889. PMCID: PMC6121255.
Article33. Li JY, Ren KK, Zhang WJ, Xiao L, Wu HY, Liu QY, Ding T, Zhang XC, Nie WJ, Ke Y, Deng KY, Liu QW, Xin HB. 2019; Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res Ther. 10:247. DOI: 10.1186/s13287-019-1366-y. PMID: 31399039. PMCID: PMC6688220.
Article34. Deng J, Bai X, Feng X, Ni J, Beretov J, Graham P, Li Y. 2019; Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer. 19:618. DOI: 10.1186/s12885-019-5824-9. PMID: 31234823. PMCID: PMC6591840.
Article35. Zhang D, Zhang P, Li L, Tang N, Huang F, Kong X, Tan X, Shi G. 2019; Irisin functions to inhibit malignant growth of human pancreatic cancer cells via downregulation of the PI3K/AKT signaling pathway. Onco Targets Ther. 12:7243–7249. DOI: 10.2147/OTT.S214260. PMID: 31564907. PMCID: PMC6732507.36. Liu J, Huang Y, Liu Y, Chen Y. 2019; Irisin enhances doxorubicin-induced cell apoptosis in pancreatic cancer by inhibiting the PI3K/AKT/NF-κB pathway. Med Sci Monit. 25:6085–6096. DOI: 10.12659/MSM.917625. PMID: 31412018. PMCID: PMC6705179.
Article37. Shi G, Tang N, Qiu J, Zhang D, Huang F, Cheng Y, Ding K, Li W, Zhang P, Tan X. 2017; Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma. Biochem Biophys Res Commun. 493:585–591. DOI: 10.1016/j.bbrc.2017.08.148. PMID: 28867187.
Article38. Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, Chen Q, Li YH, Wang JJ, Kang YM, Zhu GQ. 2015; Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci (Lond). 129:839–850. DOI: 10.1042/CS20150009. PMID: 26201094.
Article39. Bilanges B, Posor Y, Vanhaesebroeck B. 2019; PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol. 20:515–534. DOI: 10.1038/s41580-019-0129-z. PMID: 31110302.
Article40. Cheng CF, Ku HC, Lin H. 2018; PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. 19:3447. DOI: 10.3390/ijms19113447. PMID: 30400212. PMCID: PMC6274980.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cell Research in Cardiovascular System
- Stem Cells for Cardiovascular Disease
- Current Concepts in Stem Cell Therapy for Cardiovascular Diseases: What We Know and Don't Know
- MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN
- Is Stem Cell-Based Therapy Going on or Out for Cardiac Disease?