Obstet Gynecol Sci.
2021 Nov;64(6):484-495. 10.5468/ogs.21264.
17-alpha hydroxyprogesterone caproate for the prevention of recurrent preterm birth among singleton pregnant women with a prior history of preterm birth: a systematic review and meta-analysis of six randomized controlled trials
- Affiliations
-
- 1Department of Obstetrics and Gynecology, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
- 2Department of Obstetrics and Gynecology, College of Medicine, King Saud University, King Khalid University Hospital, King Saud University Medical City, Riyadh, Saudi Arabia
- 3Department of Obstetrics and Gynecology, King Fahad Medical City, Riyadh, Saudi Arabia
- 4Department of Obstetrics and Gynecology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
- 5Department of Obstetrics and Gynecology, Faculty of Medicine, Najran University, Najran, Saudi Arabia
- 6Department of Neonatology, King Fahad Medical City, Riyadh, Saudi Arabia
- 7Department of Obstetrics and Gynecology, King Saud Hospital, Unayzah, Qassim, Saudi Arabia
- 8Department of Obstetrics and Gynecology, Faculty of Medicine at Rabigh, King Abdulaziz University, Rabigh, Saudi Arabia
- 9Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
- 10Department of Obstetrics and Gynecology, Alfaisal University, Riyadh, Saudi Arabia
- 11College of Graduate Health Sciences, University of Tennessee Health Science Center, Memphis, TN, USA
- KMID: 2522472
- DOI: http://doi.org/10.5468/ogs.21264
Abstract
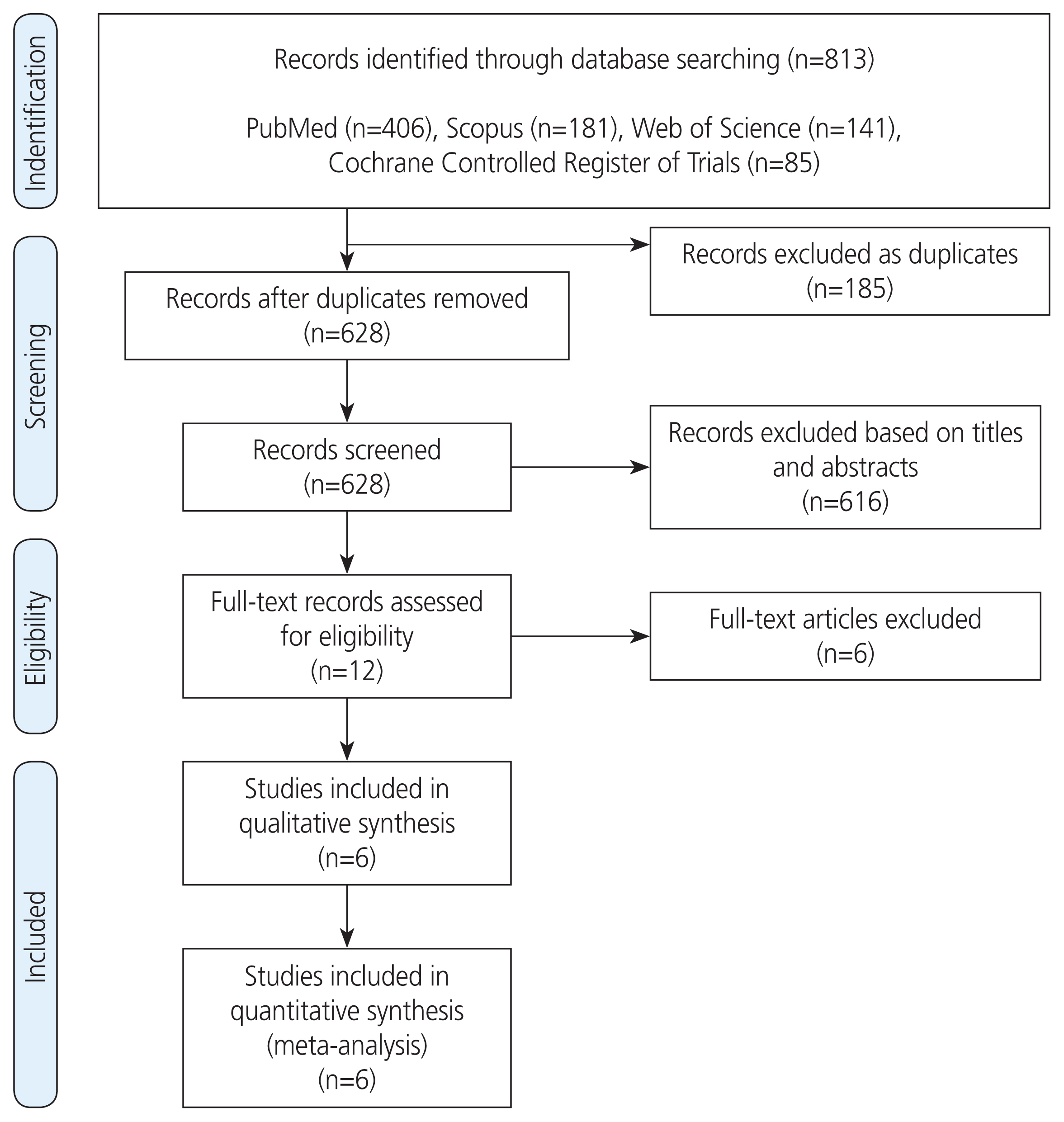
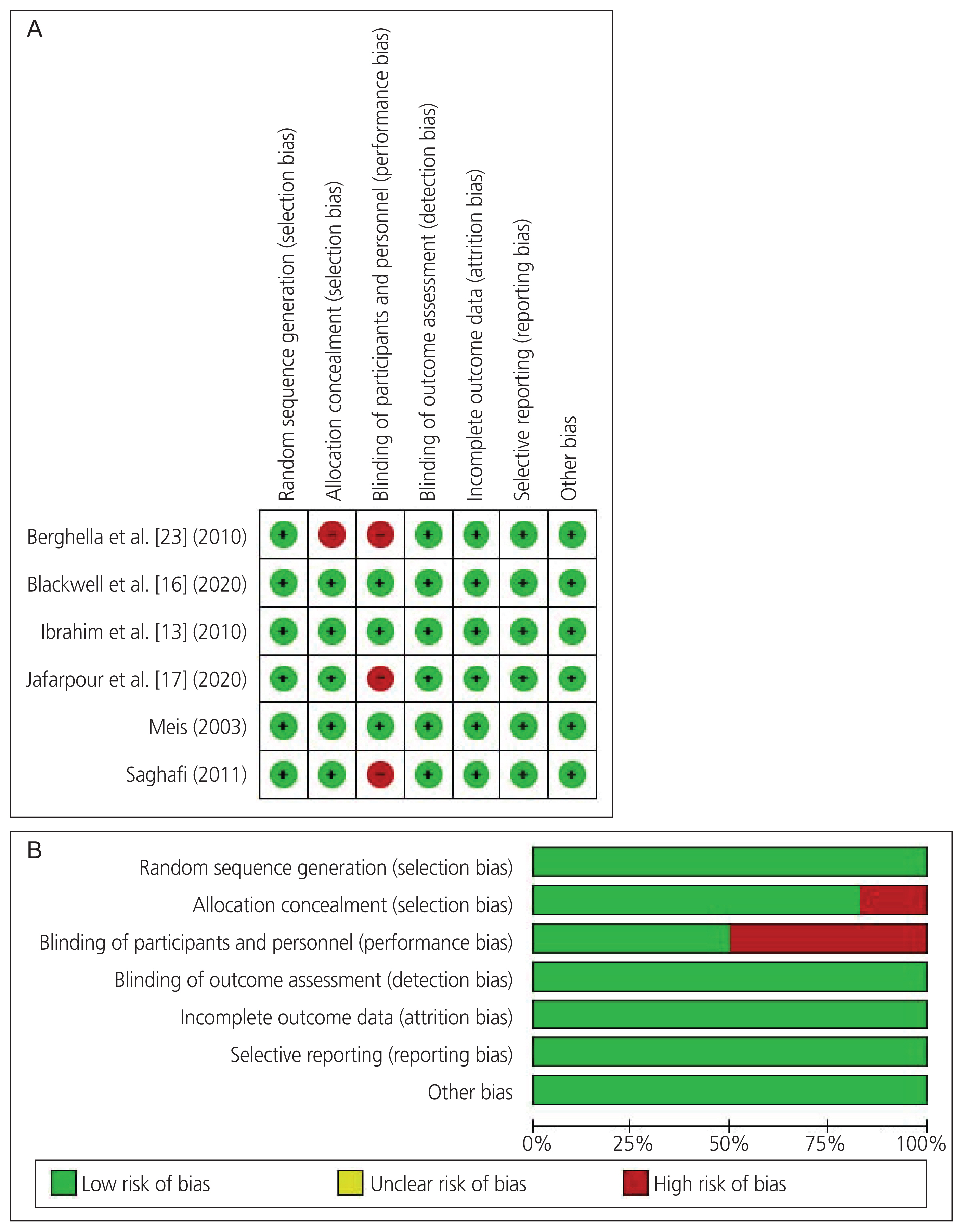
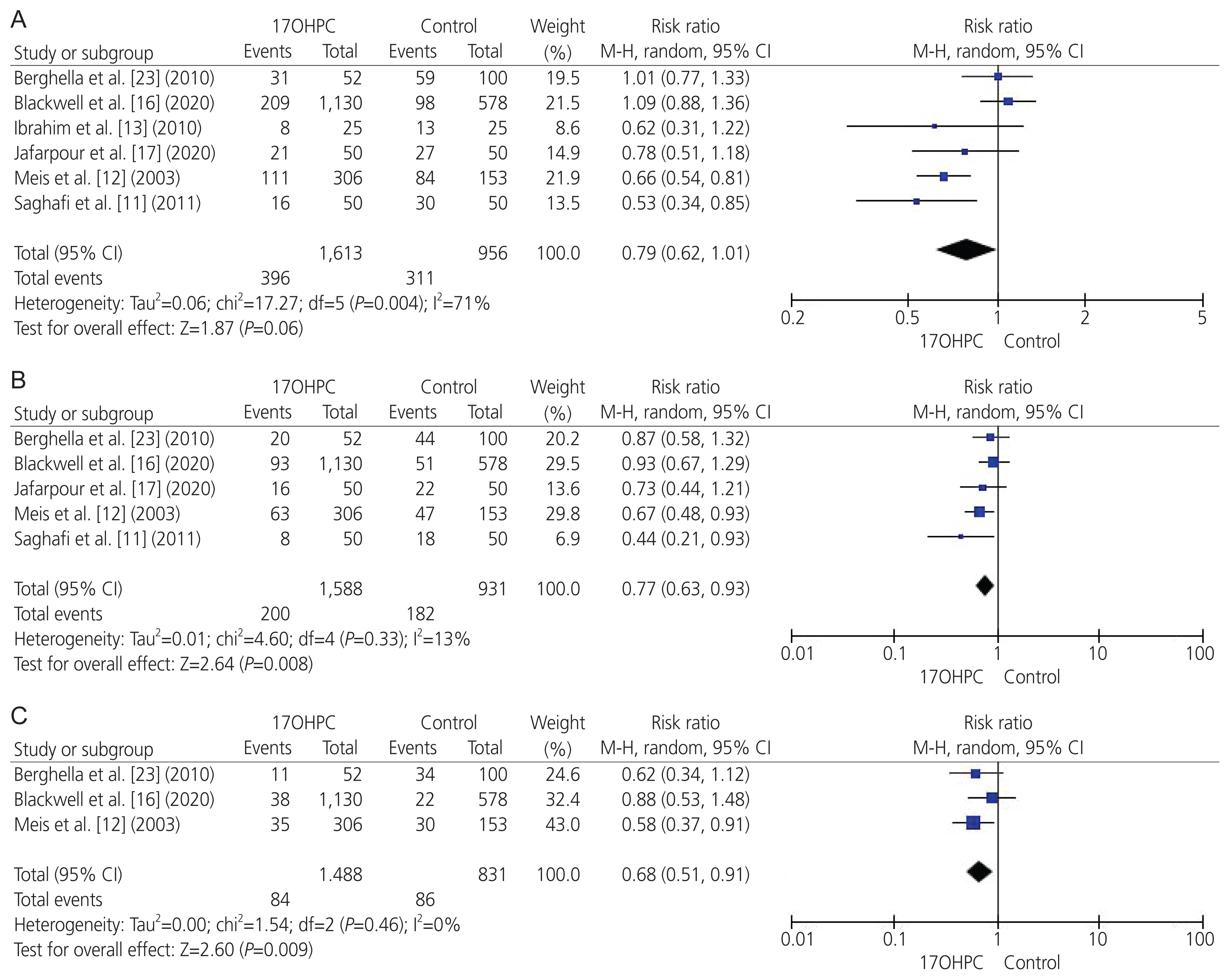
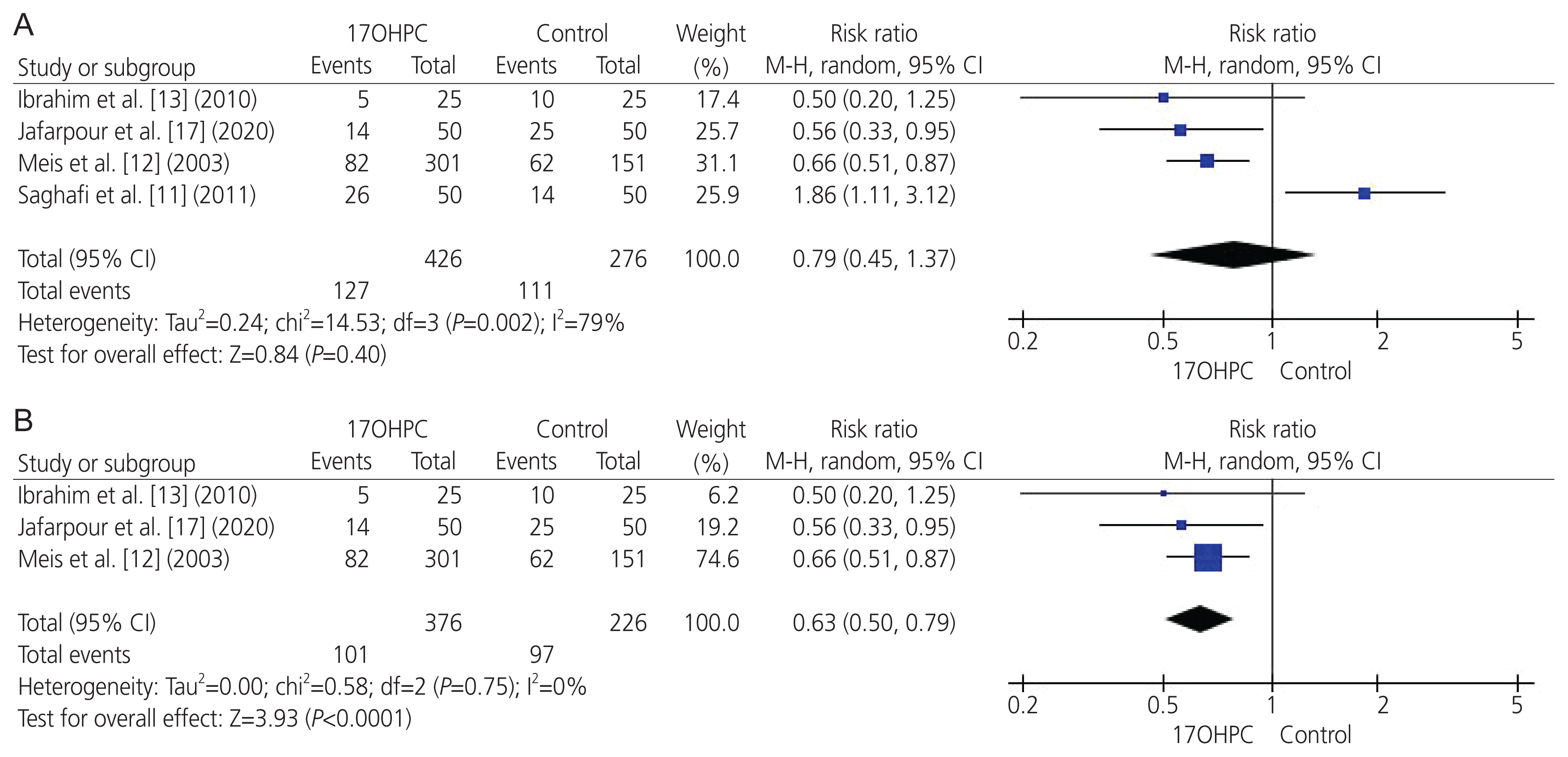
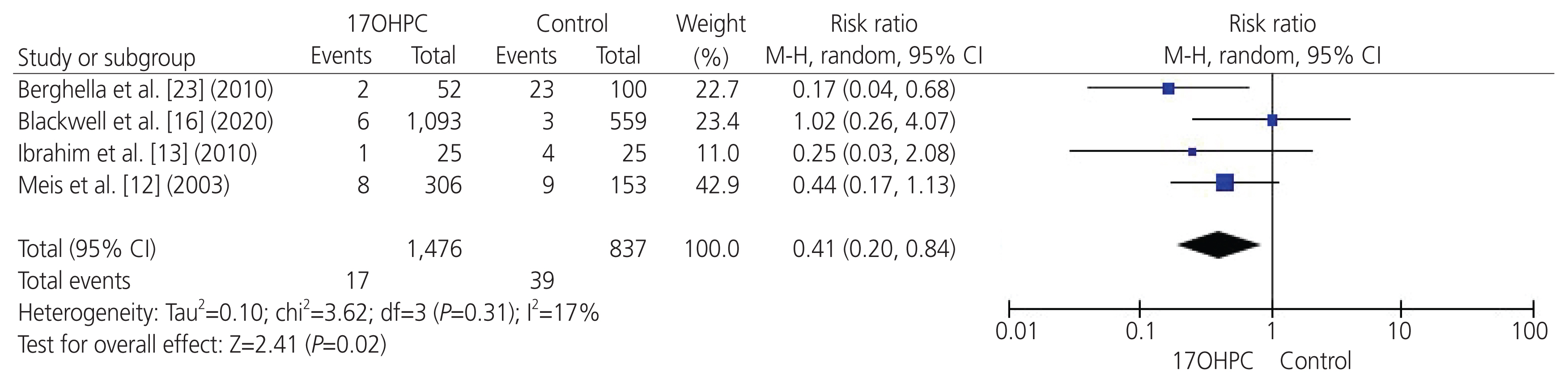
- To perform a systematic review and meta-analysis of all randomized controlled trials (RCTs) that investigated the clinical benefits of 17-alpha hydroxyprogesterone caproate (17OHPC) in the prevention of recurrent preterm birth (PTB) among singleton pregnant women with a previous history of PTB. We searched four major databases up till April 2021 and assessed the risk of bias in the included studies. We meta-analyzed various maternal-neonatal endpoints (n=18) and pooled them as mean difference or risk ratio (RR) with 95% confidence interval (CI) using the random-effects model. Six RCTs met the inclusion criteria, comprising 2,573 patients (17OHPC=1,617, control=956). RCTs revealed an overall low risk of bias. The rates of PTB <35 weeks (n=5 RCTs; RR, 0.77; 95% CI, 0.63-0.93; P=0.008), PTB <32 weeks (n=3 RCTs; RR, 0.68; 95% CI, 0.51-0.91; P=0.009), neonates with low birth weight (<2.5 kg) at delivery (n=3 RCTs; RR, 0.63; 95% CI, 0.5-0.79; P<0.001), and neonatal death (n=4 RCTs; RR, 0.41; 95% CI, 0.20-0.84; P=0.02) were significantly reduced in the 17OHPC group compared with the control group. Moreover, 17OHPC treatment correlated with a significantly decreased rate of retinopathy (n=2 RCTs; RR, 0.42; 95% CI, 0.18-0.97; P=0.004). However, there were no significant differences in the rates of neonatal intensive care unit admission, cesarean delivery, and other pretermrelated complications between both the groups. Among singleton pregnant women with a prior history of PTB, 17OHPC may favorably decrease the risks of recurrent PTB and reduce the rate of neonatal death.
Figure
Cited by 1 articles
-
Effect of autologous platelet-rich plasma for treatment of recurrent pregnancy loss: a randomized controlled trial
Leila Nazari, Saghar Salehpour, Sedighe Hosseini, Teibeh Hashemi, Nasrin Borumandnia, Elham Azizi
Obstet Gynecol Sci. 2022;65(3):266-272. doi: 10.5468/ogs.21261.
Reference
-
References
1. WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand. 1977; 56:247–53.2. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012; 379:2162–72.
Article3. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012; 379:2151–61.
Article4. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014; 345:760–5.
Article5. Esplin MS, Manuck TA, Varner MW, Christensen B, Biggio J, Bukowski R, et al. Cluster analysis of spontaneous preterm birth phenotypes identifies potential associations among preterm birth mechanisms. Am J Obstet Gynecol. 2015; 213:429.e1–9.
Article6. Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C. Prevention of preterm birth: Harnessing science to address the global epidemic. Sci Transl Med. 2014; 6:262sr5.
Article7. Serra V, Perales A, Meseguer J, Parrilla JJ, Lara C, Bellver J, et al. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: a randomised controlled double-blind multicentre trial. BJOG. 2013; 120:50–7.
Article8. Hermans FJ, Karolinski A, Othenin-Girard V, Bertolino MV, Schuit E, Salgado P, et al. Population differences and the effect of vaginal progesterone on preterm birth in women with threatened preterm labor (.). J Matern Fetal Neonatal Med. 2016; 29:3223–8.9. Tita AT, Rouse DJ. Progesterone for preterm birth prevention: an evolving intervention. Am J Obstet Gynecol. 2009; 200:219–24.
Article10. Shaamash AH, Ali MK, Attyia KM. Intramuscular 17α-hydroxyprogesterone caproate to decrease preterm delivery in women with placenta praevia: a randomised controlled trial. J Obstet Gynaecol. 2020; 40:633–8.
Article11. Saghafi N, Khadem N, Mohajeri T, Shakeri MT. Efficacy of 17α-hydroxyprogesterone caproate in prevention of preterm delivery. J Obstet Gynaecol Res. 2011; 37:1342–5.
Article12. Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003; 348:2379–85.
Article13. Ibrahim M, Mohamed Ramy AR, Younis MAF. Progesterone supplementation for prevention of preterm labor: A randomized controlled trial. Middle East Fertil Soc J. 2010; 15:39–41.
Article14. Grobman WA, Thom EA, Spong CY, Iams JD, Saade GR, Mercer BM, et al. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012; 207:390.e1–8.
Article15. American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003; 102(5 Pt 1):1115–6.16. Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, Chauhan SP, Hughes BL, Louis JM, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020; 37:127–36.
Article17. Jafarpour H, Mousavi SJ, Mirghorbani M, Razavi AR, Atarod Z. Effect of 17 α-hydroxyprogesterone caproate on the prevention of preterm labor: a randomized controlled trial study. J Midwifery Reproductive Health. 2020; 8:2317–23.18. Fernandez-Macias R, Martinez-Portilla RJ, Cerrillos L, Figueras F, Palacio M. A systematic review and meta-analysis of randomized controlled trials comparing 17-alpha-hydroxyprogesterone caproate versus placebo for the prevention of recurrent preterm birth. Int J Gynaecol Obstet. 2019; 147:156–64.19. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.
Article20. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 [Internet]. London (UK): Cochrane;c2021. [cited 2021 May 13]. Available from: www.training.cochrane.org/handbook .21. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
Article23. Berghella V, Figueroa D, Szychowski JM, Owen J, Hankins GD, Iams JD, et al. 17-alpha-hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol. 2010; 202:351.e1–6.
Article24. Pirjani R, Heidari R, Rahimi-Foroushani A, Bayesh S, Esmailzadeh A. 17-alpha-hydroxyprogesterone caproate versus vaginal progesterone suppository for the prevention of preterm birth in women with a sonographically short cervix: a randomized controlled trial. J Obstet Gynaecol Res. 2017; 43:57–64.
Article25. Choi SJ, Kwak DW, Kil K, Kim SC, Kwon JY, Kim YH, et al. Vaginal compared with intramuscular progestogen for preventing preterm birth in high-risk pregnant women (VICTORIA study): a multicentre, open-label randomised trial and meta-analysis. BJOG. 2020; 127:1646–54.
Article26. Thain S, Yeo GSH, Kwek K, Chern B, Tan KH. Spontaneous preterm birth and cervical length in a pregnant Asian population. PLoS One. 2020; 15:e0230125.
Article27. Carter EB, Cahill AG, Olsen MA, Macones GA, Tuuli MG, Stout MJ. Practical considerations with 17-Hydroxyprogesterone caproate for preterm birth prevention: does timing of initiation and compliance matter? J Perinatol. 2019; 39:1182–9.
Article28. Ning A, Vladutiu CJ, Dotters-Katz SK, Goodnight WH, Manuck TA. Gestational age at initiation of 17-alpha hydroxyprogesterone caproate and recurrent preterm birth. Am J Obstet Gynecol. 2017; 217:371.e1–371.e7.
Article29. MAKENA® (hydroxyprogesterone caproate injection) for intramuscular or subcutaneous use [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;c2018. [cited 2021 Sep 18]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf .30. Sibai B, Saade GR, Das AF, Gudeman J. Safety review of hydroxyprogesterone caproate in women with a history of spontaneous preterm birth. J Perinatol. 2021; 41:718–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Progesterone treatment for the prevention of preterm birth
- Progesterone Treatment for Prevention of Preterm Birth
- Use of progesterone supplement therapy for prevention of preterm birth: review of literatures
- Progesterone for the Prevention of Preterm Birth
- How to screen the cervix and reduce the risk of spontaneous preterm birth in asymptomatic women without a prior preterm birth