J Pathol Transl Med.
2021 Nov;55(6):369-379. 10.4132/jptm.2021.08.03.
Post-mortem assessment of vimentin expression as a biomarker for renal tubular regeneration following acute kidney injury
- Affiliations
-
- 1Arkadi M. Rywlin M.D. Department of Pathology and Laboratory Medicine, Mount Sinai Medical Center, Miami Beach, FL, USA
- 2Department of Internal Medicine, Mount Sinai Medical Center, Miami Beach, FL, USA
- 3Department of Mathematics, Faculty of Sciences, Lebanese University, Nabatieh, Lebanon
- 4Herbert Wertheim College of Medicine, Florida International University, Miami, FL, USA
- KMID: 2522438
- DOI: http://doi.org/10.4132/jptm.2021.08.03
Abstract
- Background
Acute kidney injury (AKI) is a common cause of morbidity and mortality. It mainly targets the renal tubular epithelium with pathological changes, referred to as acute tubular injury. The latter is followed by a regenerative response that is difficult to visualize on routine hematoxylin and eosin (H&E) stains. In this study, we examined the regenerative capacity of renal tubules by correlating vimentin (VIM) immunohistochemical (IHC) expression and pathological findings of AKI and renal tubular regeneration (RTR) on H&E.
Methods
We reviewed 23 autopsies performed in the clinical setting of AKI and RTR. VIM expression was scored in the renal cortical tubular epithelium using a statistical cutoff ≥ 3% for high expression and < 3% for low expression.
Results
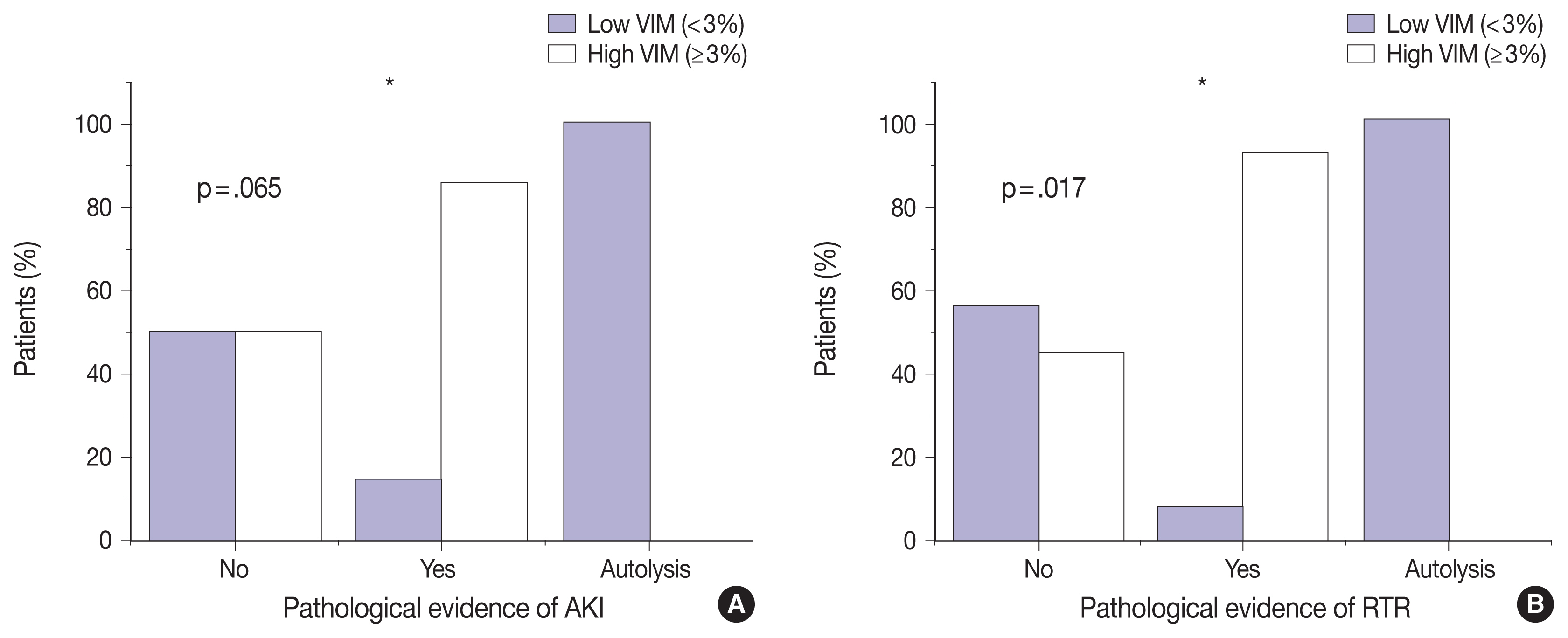
Of the 23 kidney tissues examined, seven (30.4%) had low VIM expression, and 16 (69.6%) had high VIM expression. Kidney tissues with evidence of AKI and RTR had significantly higher VIM expression. Renal peritubular microenvironment features showing regenerative changes on H&E were associated with high VIM expression. In the univariate model, kidney tissues with RTR were 18-fold more likely to have high VIM expression.
Conclusions
In conclusion, our findings suggest that VIM could serve as an IHC marker for RTR following AKI. However, correlation with H&E findings remains critical to excluding chronic tubular damage. Collectively, our preliminary results pave the way for future studies including a larger sample size to validate the use of VIM as a reliable biomarker for RTR.
Keyword
Figure
Reference
-
References
1. Eknoyan G, Agodoa L. On improving outcomes and quality of dialysis care, and more. Am J Kidney Dis. 2002; 39:889–91.
Article2. Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016; 37:85–98.3. Akcay A, Turkmen K, Lee D, Edelstein CL. Update on the diagnosis and management of acute kidney injury. Int J Nephrol Renovasc Dis. 2010; 3:129–40.4. Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012; 2:1303–53.
Article5. Sawhney S, Fraser SD. Epidemiology of AKI: utilizing large databases to determine the burden of AKI. Adv Chronic Kidney Dis. 2017; 24:194–204.
Article6. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015; 87:46–61.
Article7. Hoste EA, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018; 14:607–25.
Article8. Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. 2019; 79:365–79.
Article9. Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS. Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation. 2001; 104:1985–91.10. Rosen S, Stillman IE. Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol. 2008; 19:871–5.
Article11. Berger K, Moeller MJ. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin Nephrol. 2014; 34:394–403.
Article12. Wen Y, Yang C, Menez SP, Rosenberg AZ, Parikh CR. A systematic review of clinical characteristics and histologic descriptions of acute tubular injury. Kidney Int Rep. 2020; 5:1993–2001.
Article13. Toback FG. Regeneration after acute tubular necrosis. Kidney Int. 1992; 41:226–46.
Article14. Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003; 14(Suppl 1):S55–61.
Article15. Andrianova NV, Buyan MI, Zorova LD, et al. Kidney cells regeneration: dedifferentiation of tubular epithelium, resident stem cells and possible niches for renal progenitors. Int J Mol Sci. 2019; 20:6326.
Article16. Solez K, Morel-Maroger L, Sraer JD. The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore). 1979; 58:362–76.17. Molitoris BA, Hoilien CA, Dahl R, Ahnen DJ, Wilson PD, Kim J. Characterization of ischemia-induced loss of epithelial polarity. J Membr Biol. 1988; 106:233–42.
Article18. Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994; 93:2175–88.
Article19. Fujigaki Y, Goto T, Sakakima M, et al. Kinetics and characterization of initially regenerating proximal tubules in S3 segment in response to various degrees of acute tubular injury. Nephrol Dial Transplant. 2006; 21:41–50.
Article20. Frazier KS, Seely JC, Hard GC, et al. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol. 2012; 40(4 Suppl):14S–86S.
Article21. Schena FP. Role of growth factors in acute renal failure. Kidney Int Suppl. 1998; 66:S11–5.22. Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab. 2003; 80:365–76.
Article23. Joh K, Muso E, Shigematsu H, et al. Renal pathology of ANCA-related vasculitis: proposal for standardization of pathological diagnosis in Japan. Clin Exp Nephrol. 2008; 12:277–91.
Article24. Textor SC. Pathophysiology of renal artery disease. Creager MA, Beckman JA, Loscalzo J, editors. Vascular medicine: a companion to Braunwald’s heart disease. 2nd ed.Philadelphia: W.B. Saunders;2013. p. 285–95.
Article25. Breshears MA, Confer AW. The urinary system. Zachary JF, editor. Pathologic basis of veterinary disease. St. Louis: Elsevier;2017. p. 617–81.
Article26. Mehendiratta M, Bishen KA, Boaz K, Mathias Y. Ghost cells: a journey in the dark. Dent Res J (Isfahan). 2012; 9(Suppl 1):S1–8.27. Gonlusen G, Ergin M, Paydas S, Tunali N. The expression of cytoskeletal proteins (alpha-SMA, vimentin, desmin) in kidney tissue: a comparison of fetal, normal kidneys, and glomerulonephritis. Int Urol Nephrol. 2001; 33:299–305.28. Inker LA, Shaffi K, Levey AS. Estimating glomerular filtration rate using the chronic kidney disease-epidemiology collaboration creatinine equation: better risk predictions. Circ Heart Fail. 2012; 5:303–6.29. Smeets B, Boor P, Dijkman H, et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol. 2013; 229:645–59.
Article30. Robinson-Bennett B, Han A. Role of immunohistochemistry in elucidating lung cancer metastatic to the ovary from primary ovarian carcinoma. Hayat MA, editor. Handbook of immunohistochemistry and in situ hybridization of human carcinomas. Amsterdam: Elsevier Academic Press;2006. p. 537–45.31. Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelial-mesenchymal transition in human breast cancer: observations in vitro and in vivo. Cells Tissues Organs. 2007; 185:191–203.32. Holthofer H, Miettinen A, Lehto VP, Lehtonen E, Virtanen I. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult human kidneys. Lab Invest. 1984; 50:552–9.33. Mezni I, Xu-Dubois Y, Galichon P, et al. Tubular expression of vimentin as a biomarker of kidney graft fibrogenesis: invasive and noninvasive measurements: abstract# A22. Transplantation. 2014; 98:877.34. Vogetseder A, Karadeniz A, Kaissling B, Le Hir M. Tubular cell proliferation in the healthy rat kidney. Histochem Cell Biol. 2005; 124:97–104.
Article35. Chambers BE, Wingert RA. Renal progenitors: roles in kidney disease and regeneration. World J Stem Cells. 2016; 8:367–75.
Article36. Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003; 14:3138–46.
Article37. Kitamura S, Yamasaki Y, Kinomura M, et al. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005; 19:1789–97.
Article38. Bussolati B, Bruno S, Grange C, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005; 166:545–55.
Article39. Kim K, Lee KM, Han DJ, Yu E, Cho YM. Adult stem cell-like tubular cells reside in the corticomedullary junction of the kidney. Int J Clin Exp Pathol. 2008; 1:232–41.40. Cheaito KA, Bahmad HF, Hadadeh O, et al. EMT markers in locally-advanced prostate cancer: predicting recurrence? Front Oncol. 2019; 9:131.
Article41. Hansson J, Hultenby K, Cramnert C, et al. Evidence for a morphologically distinct and functionally robust cell type in the proximal tubules of human kidney. Hum Pathol. 2014; 45:382–93.
Article42. Vansthertem D, Caron N, Decleves AE, et al. Label-retaining cells and tubular regeneration in postischaemic kidney. Nephrol Dial Transplant. 2008; 23:3786–97.
Article43. Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014; 9:157–79.
Article44. Lee SJ, Yoo JD, Choi SY, Kwon OS. The expression and secretion of vimentin in the progression of non-alcoholic steatohepatitis. BMB Rep. 2014; 47:457–62.
Article45. Li FJ, Surolia R, Li H, et al. Autoimmunity to vimentin is associated with outcomes of patients with idiopathic pulmonary fibrosis. J Immunol. 2017; 199:1596–605.
Article46. Danielsson F, Peterson MK, Caldeira Araujo H, Lautenschlager F, Gad AK. Vimentin diversity in health and disease. Cells. 2018; 7:147.
Article47. CAP Policy Manual: Policy PP Minimum period of retention of laboratory records and materials. Northfield: College of American Pathologists;2020.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Fas Ligand (FasL) in Normal Kidney and Experimental Acute Tubular Necrosis
- Intra-renal slow cell-cycle cells contribute to the restoration of kidney tubules injured by ischemia/reperfusion
- Expression of Platelet Derived Growth Factor-A, C and Platelet Derived Growth Factor Receptor-alpha in the Ischemia Reperfusion Renal Failure Model
- Apoptosis and Cell Proliferation in Experimental Acute Tubular Necrosis Induced by Intramuscular Glycerol Injection
- Acute Tubular Necrosis Associated with Typhoid Fever