J Korean Med Sci.
2021 Nov;36(42):e271. 10.3346/jkms.2021.36.e271.
Safety and Effectiveness of F-18 Fluoroestradiol Positron Emission Tomography/Computed Tomography: a Systematic Review and Meta-analysis
- Affiliations
-
- 1Department of New Health Technology Assessment Research, National Evidence-based Healthcare Collaborating Agency, Seoul, Korea
- KMID: 2522116
- DOI: http://doi.org/10.3346/jkms.2021.36.e271
Abstract
- Background
We conducted a pooled analysis of the diagnostic accuracy of F-18 fluoroestradiol (F-18 FES) positron emission tomography/computed tomography (PET/CT) assessing estrogen receptor expression of patients who have recurrent or metastatic breast cancer.
Methods
Two investigators and seven related experts (from the departments of nuclear medicine, hematological oncology, surgery, and evidence-based medicine) evaluated the effectiveness of F-18 FES PET/CT according to diagnostic accuracy and correlation with immunohistochemistry tests via systematic literature review, and safety according to test-related side effects. The present study was conducted in accordance with the Scottish Intercollegiate Guidelines (SIGN), and the Cochrane, and Preferred Reporting Items for Systematic Reviews and Meta Analyses guidelines. The SIGN tools were used for quality assessment.
Results
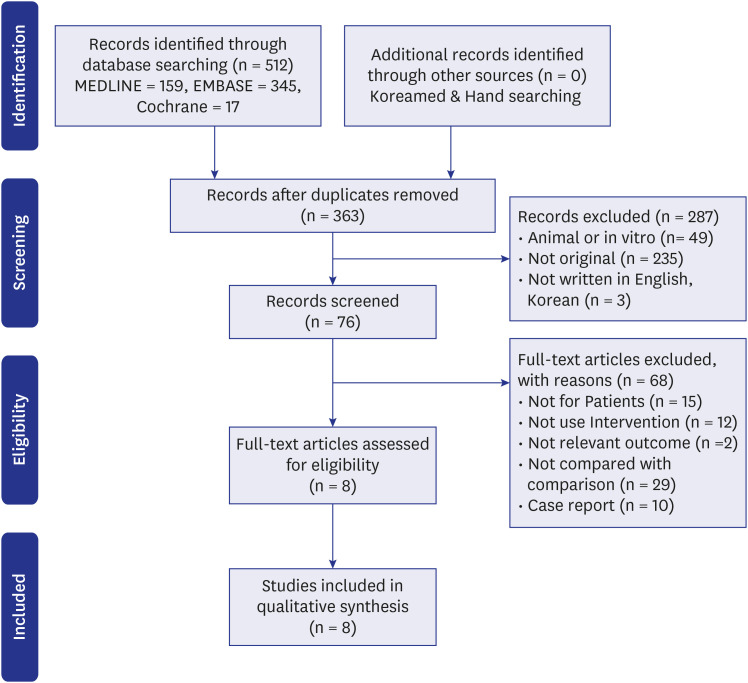
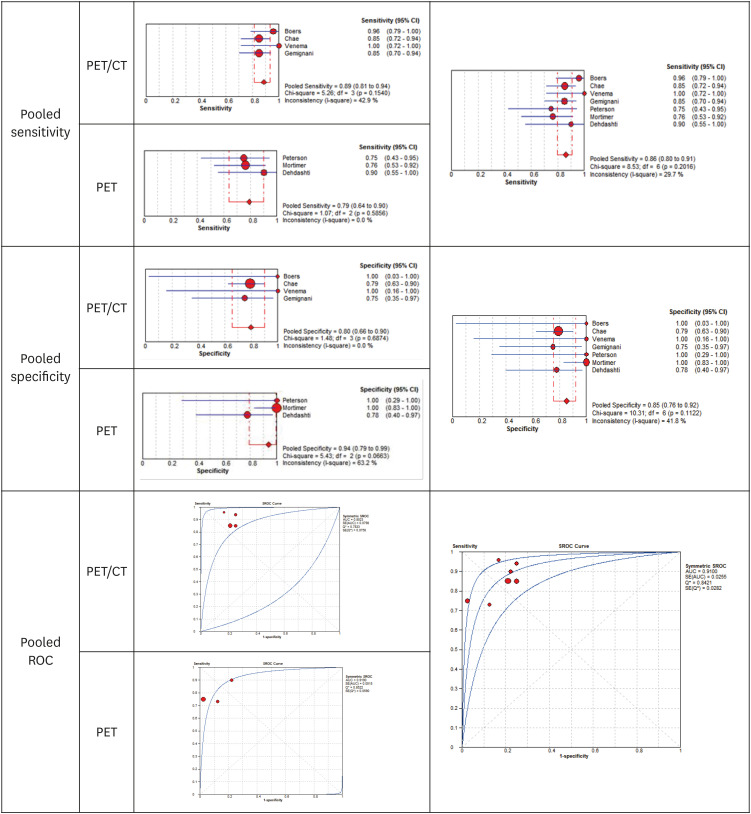
Of the 512 articles retrieved in the literature search, 8 were deemed to be eligible for inclusion. Results of the evaluation indicated that the F-18 FES PET/CT test was safe because patients who reported pain in the injection site in the analyzed articles are most likely to be caused by mechanical injury from needle injection not by administration of radioactive materials. Assessment of diagnostic accuracy based on data from seven studies revealed a pooled sensitivity and specificity of 0.86 and 0.85, respectively.
Conclusion
As such, the test was evaluated to be a safe and effective and, considering the anatomical site where only invasive tests are possible, the test was deemed to have high clinical utility.
Figure
Reference
-
1. Korean Breast Cancer Society. Breast Cancer Facts & Figures 2020. Seoul, Korea: Korean Breast Cancer Society;2020.2. National cancer information. Updated 2021. Accessed February 15, 2021. https://www.cancer.go.kr/.3. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011; 378(9793):771–784. PMID: 21802721.4. Vollenweider-Zerargui L, Barrelet L, Wong Y, Lemarchand-Béraud T, Gómez F. The predictive value of estrogen and progesterone receptors' concentrations on the clinical behavior of breast cancer in women. Clinical correlation on 547 patients. Cancer. 1986; 57(6):1171–1180. PMID: 3943040.
Article5. Osborne CK, Yochmowitz MG, Knight WA 3rd, McGuire WL. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980; 46(12):Suppl. 2884–2888. PMID: 7448733.
Article6. Wittliff JL. Steroid-hormone receptors in breast cancer. Cancer. 1983; 53(S3):630–643.
Article7. Pertschuk LP, Kim DS, Nayer K, Feldman JG, Eisenberg KB, Carter AC, et al. Immunocytochemical estrogen and progestin receptor assays in breast cancer with monoclonal antibodies. Histopathologic, demographic, and biochemical correlations and relationship to endocrine response and survival. Cancer. 1990; 66(8):1663–1670. PMID: 2208020.
Article8. Hähnel R, Woodings T, Vivian AB. Prognostic value of estrogen receptors in primary breast cancer. Cancer. 1979; 44(2):671–675. PMID: 476576.
Article9. Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957; 11(3):359–377. PMID: 13499785.10. Chang ES, Park KK, Kim YS. Detection of estrogen receptors with monoclonal antibodies in formalin-fixed; paraffin-embedded breast cancer tissue* (comparison of immunohistochemical method with ER - D5 antibody and ER-ICA). Keimyung Med J. 1990; 9:322–322.11. Mirecki DM, Jordon VC. Steroid hormone receptors and human breast cancer. Lab Med. 1985; 16(5):287–294.
Article12. Taylor CR, Cooper CL, Kurman RJ, Goebelsmann U, Markland FS Jr. Detection of estrogen receptor in breast and endometrial carcinoma by the immunoperoxidase technique. Cancer. 1981; 47(11):2634–2640. PMID: 6167343.
Article13. Fisher ER, Anderson S, Dean S, Dabbs D, Fisher B, Siderits R, et al. Solving the dilemma of the immunohistochemical and other methods used for scoring estrogen receptor and progesterone receptor in patients with invasive breast carcinoma. Cancer. 2005; 103(1):164–173. PMID: 15565575.
Article14. Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020; 38(12):1346–1366. PMID: 31928404.
Article15. Castagnetta L, Traina A, Di Carlo A, Carruba G, Lo Casto M, Mesiti M, et al. Do multiple oestrogen receptor assays give significant additional information for the management of breast cancer? Br J Cancer. 1989; 59(4):636–638. PMID: 2713250.
Article16. Korean Society of Breast Imaging. Breast Diagnostic Imaging. Seoul, Korea: Ilchokak;2011.17. Mintun MA, Welch MJ, Siegel BA, Mathias CJ, Brodack JW, McGuire AH, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988; 169(1):45–48. PMID: 3262228.
Article18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339:b2535. PMID: 19622551.
Article19. Scottish Intercollegiate Guidelines Network. Improving patient care through evidence-based guidelines. Updated 2021. Accessed February 15, 2021. http://www.sign.ac.uk.20. Boers J, Venema CM, de Vries EF, Glaudemans AW, Kwee TC, Schuuring E, et al. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur J Cancer. 2020; 126:11–20. PMID: 31891878.
Article21. Chae SY, Ahn SH, Kim SB, Han S, Lee SH, Oh SJ, et al. Diagnostic accuracy and safety of 16α-[18F]fluoro-17β-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: a prospective cohort study. Lancet Oncol. 2019; 20(4):546–555. PMID: 30846327.22. Dehdashti F, Mortimer JE, Siegel BA, Griffeth LK, Bonasera TJ, Fusselman MJ, et al. Positron tomographic assessment of estrogen receptors in breast cancer: comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995; 36(10):1766–1774. PMID: 7562040.23. Gemignani ML, Patil S, Seshan VE, Sampson M, Humm JL, Lewis JS, et al. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J Nucl Med. 2013; 54(10):1697–1702. PMID: 23970364.
Article24. Mortimer JE, Dehdashti F, Siegel BA, Katzenellenbogen JA, Fracasso P, Welch MJ. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: correlation with estrogen receptor status and response to systemic therapy. Clin Cancer Res. 1996; 2(6):933–939. PMID: 9816253.25. Peterson LM, Kurland BF, Schubert EK, Link JM, Gadi VK, Specht JM, et al. A phase 2 study of 16α-[18F]-fluoro-17β-estradiol positron emission tomography (FES-PET) as a marker of hormone sensitivity in metastatic breast cancer (MBC). Mol Imaging Biol. 2014; 16(3):431–440. PMID: 24170452.
Article26. Venema CM, Mammatas LH, Schröder CP, van Kruchten M, Apollonio G, Glaudemans AW, et al. Androgen and estrogen receptor imaging in metastatic breast cancer patients as a surrogate for tissue biopsies. J Nucl Med. 2017; 58(12):1906–1912. PMID: 28912144.
Article27. Yang Z, Sun Y, Xu X, Zhang Y, Zhang J, Xue J, et al. The assessment of estrogen receptor status and its intratumoral heterogeneity in patients with breast cancer by using 18F-fluoroestradiol PET/CT. Clin Nucl Med. 2017; 42(6):421–427. PMID: 28221191.
Article28. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th ed. New York, NY, USA: Springer;2017.29. Arimappamagan A, Kadambari D, Srinivasan K, Krishnan R, Elangovan S, Reddy KS. Complete axillary conversion after neoadjuvant chemotherapy in locally advanced breast cancer: a step towards conserving axilla? Indian J Cancer. 2004; 41(1):13–17. PMID: 15105574.30. Hieken TJ, Boughey JC, Jones KN, Shah SS, Glazebrook KN. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol. 2013; 20(10):3199–3204. PMID: 23846781.
Article31. Di Micco R, Zuber V, Fiacco E, Carriero F, Gattuso MI, Nazzaro L, et al. Sentinel node biopsy after primary systemic therapy in node positive breast cancer patients: time trend, imaging staging power and nodal downstaging according to molecular subtype. Eur J Surg Oncol. 2019; 45(6):969–975. PMID: 30744944.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 18F-FDOPA PET/CT in Oncology: Procedural Guideline by the Korean Society of Nuclear Medicine
- The Usefulness of 18 F-FDG PET to Differentiate Subtypes of Dementia: The Systematic Review and Meta-Analysis
- F-18 fluorodeoxyglucose positron emission tomography/computed tomography in the infection of heart
- 18F-2-Deoxy-2-Fluoro-D-Glucose Positron Emission Tomography: Computed Tomography for Preoperative Staging in Gastric Cancer Patients
- PET Imaging for Gynecologic Malignancy