Korean J Physiol Pharmacol.
2021 Nov;25(6):545-553. 10.4196/kjpp.2021.25.6.545.
An experience on the model-based evaluation of pharmacokinetic drug-drug interaction for a long half-life drug
- Affiliations
-
- 1PIPET (Pharmacometrics Institute for Practical Education and Training), College of Medicine, The Catholic University of Korea, Korea
- 2Department of Pharmacology, College of Medicine, The Catholic University of Korea, 3 Q-fitter, Inc., Seoul 06591, Korea
- KMID: 2521480
- DOI: http://doi.org/10.4196/kjpp.2021.25.6.545
Abstract
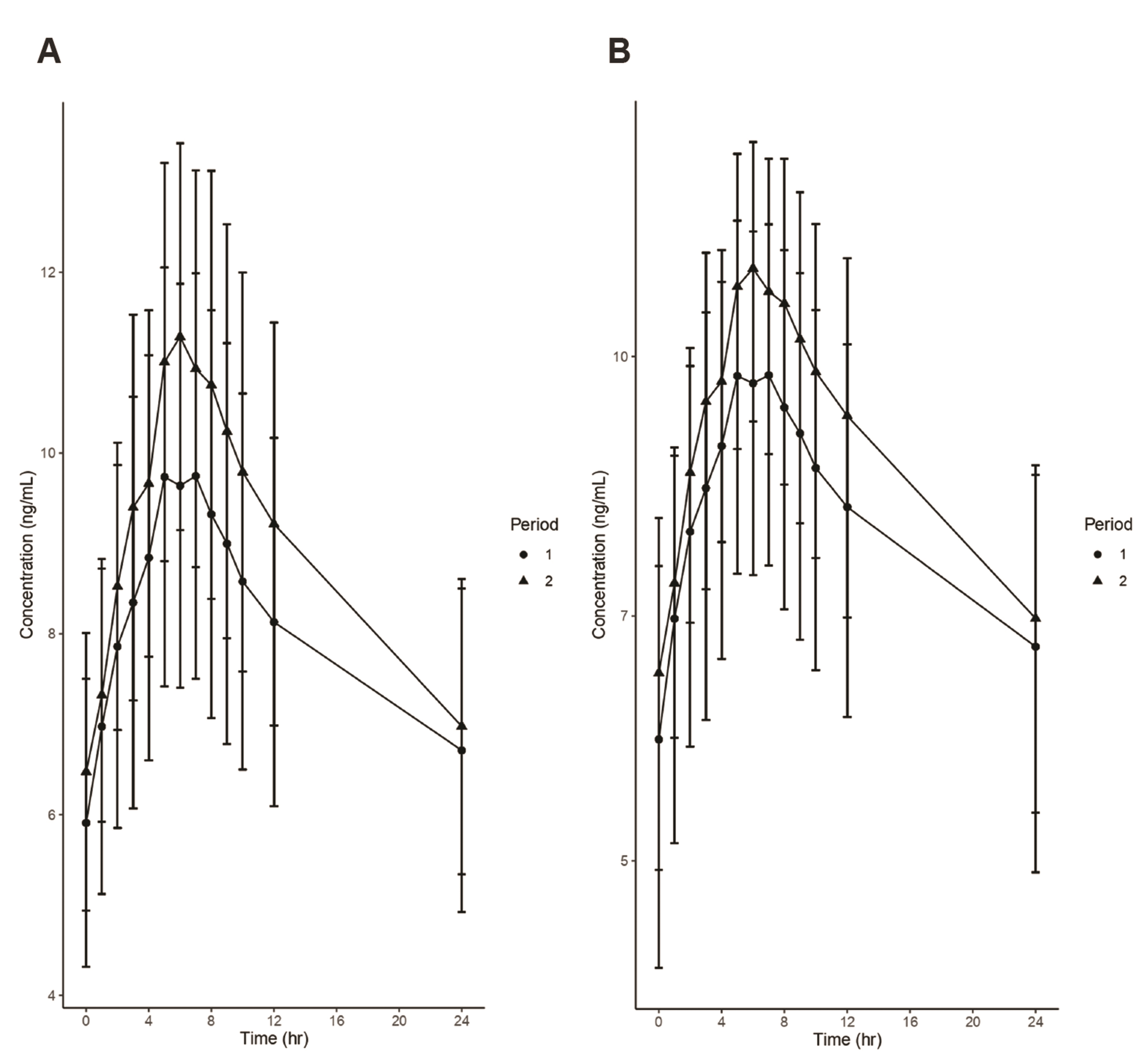
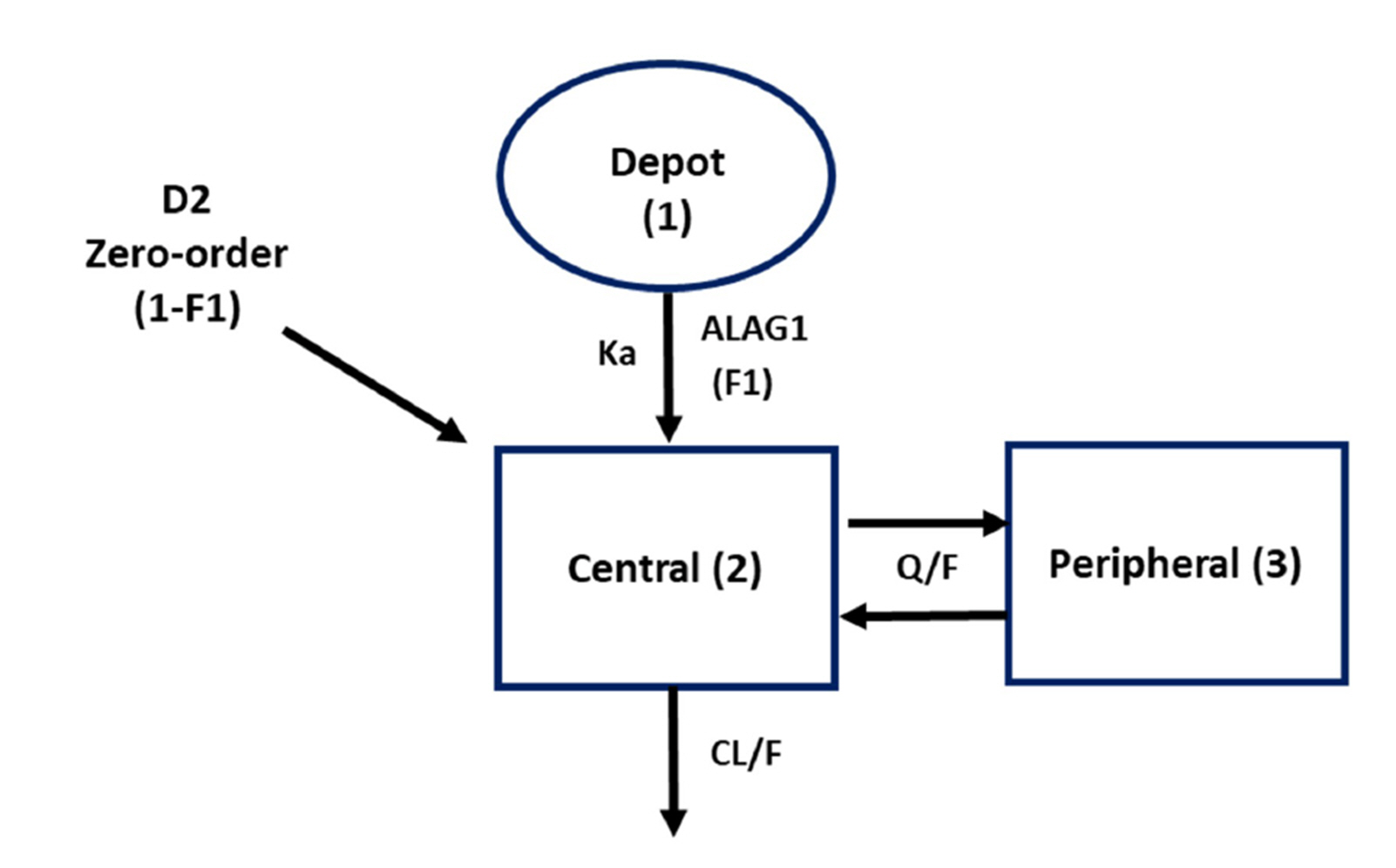
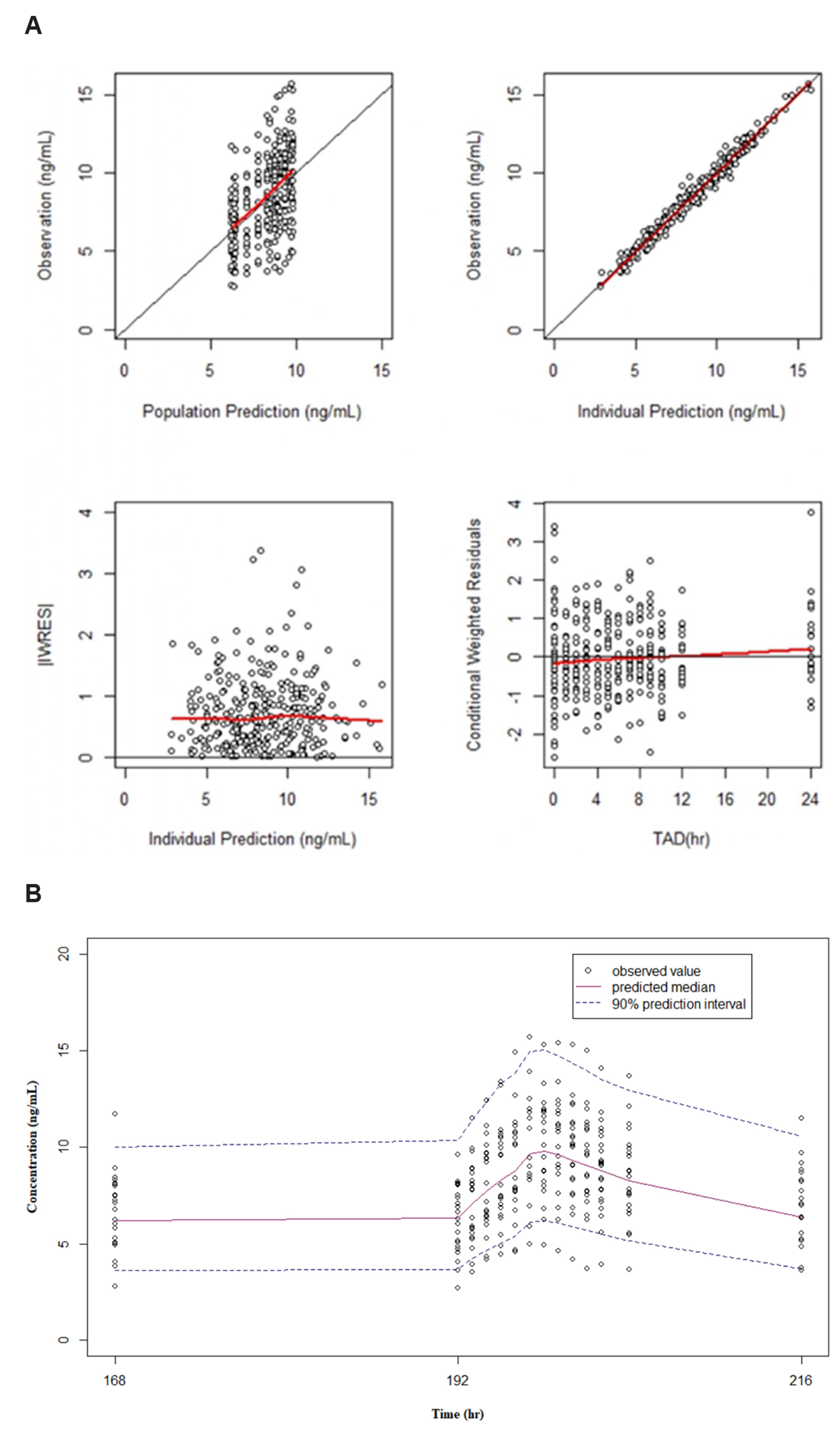
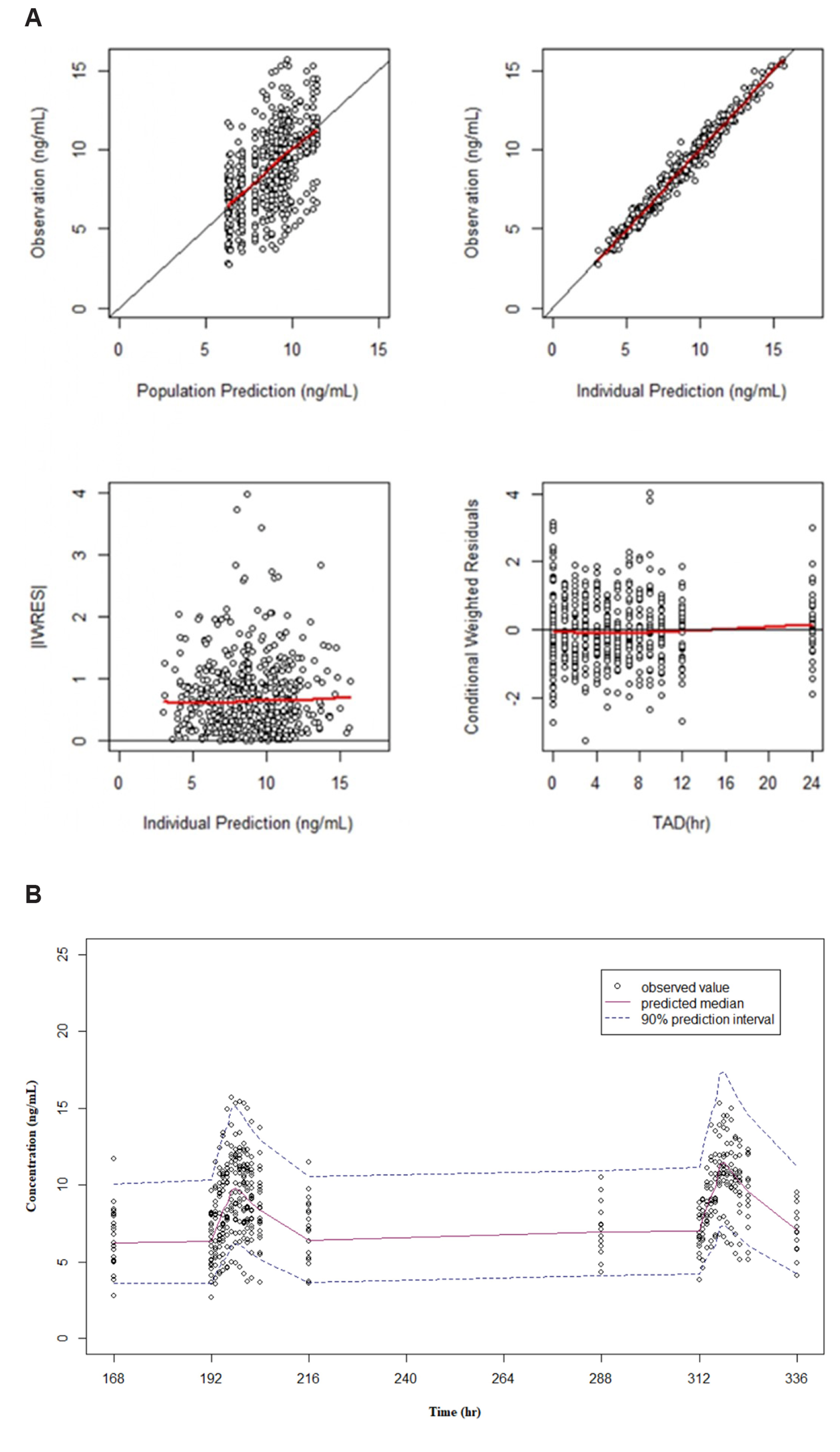
- Fixed-dose combinations development requires pharmacokinetic drugdrug interaction (DDI) studies between active ingredients. For some drugs, pharmacokinetic properties such as long half-life or delayed distribution, make it difficult to conduct such clinical trials and to estimate the exact magnitude of DDI. In this study, the conventional (non-compartmental analysis and bioequivalence [BE]) and modelbased analyses were compared for their performance to evaluate DDI using amlodipine as an example. Raw data without DDI or simulated data using pharmacokinetic models were compared to the data obtained after concomitant administration. Regardless of the methodology, all the results fell within the classical BE limit. It was shown that the model-based approach may be valid as the conventional approach and reduce the possibility of DDI overestimation. Several advantages (i.e., quantitative changes in parameters and precision of confidence interval) of the model-based approach were demonstrated, and possible application methods were proposed. Therefore, it is expected that the model-based analysis is appropriately utilized according to the situation and purpose.
Figure
Reference
-
1. Düsing R. 2010; Optimizing blood pressure control through the use of fixed combinations. Vasc Health Risk Manag. 6:321–325. DOI: 10.2147/VHRM.S9989. PMID: 20531950. PMCID: PMC2879293.
Article2. Lin CP, Tung YC, Hsiao FC, Yang CH, Kao YW, Lin YS, Chu YC, Chu PH. 2020; Fixed-dose combination of amlodipine and atorvastatin improves clinical outcomes in patients with concomitant hypertension and dyslipidemia. J Clin Hypertens (Greenwich). 22:1846–1853. DOI: 10.1111/jch.14016. PMID: 32862551.
Article3. Simons LA, Chung E, Ortiz M. 2017; Long-term persistence with single-pill, fixed-dose combination therapy versus two pills of amlodipine and perindopril for hypertension: Australian experience. Curr Med Res Opin. 33:1783–1787. DOI: 10.1080/03007995.2017.1367275. PMID: 28805468.
Article4. U.S. Food and Drug Administration. 2020. Amlodipine prescribing information [Internet]. U.S. Food and Drug Administration;Silver Spring: Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211340s000lbl.pdf. cited 2020 Nov 20.5. U.S. Food and Drug Administration. 2020. Clinical drug interaction studies - cytochrome P450 enzyme- and transporter-mediated drug interactions guidance for industry [Internet]. U.S. Food and Drug Administration;Silver Spring: Available from: https://www.fda.gov/media/134581/download. cited 2020 Nov 20.6. Lehr T, Staab A, Trommeshauser D, Schaefer HG, Kloft C. 2010; Semi-mechanistic population pharmacokinetic drug-drug interaction modelling of a long half-life substrate and itraconazole. Clin Pharmacokinet. 49:53–66. DOI: 10.2165/11317210-000000000-00000. PMID: 20000889.
Article7. Svensson EM, Acharya C, Clauson B, Dooley KE, Karlsson MO. 2016; Pharmacokinetic interactions for drugs with a long half-life-evidence for the need of model-based analysis. AAPS J. 18:171–179. DOI: 10.1208/s12248-015-9829-2. PMID: 26463060. PMCID: PMC4706279.
Article8. Choi S, Jeon S, Yim DS, Han S. 2020; Contribution of trough concentration data in the evaluation of multiple-dose pharmacokinetics for drugs with delayed distributional equilibrium and long half-life. Drug Des Devel Ther. 14:811–821. DOI: 10.2147/DDDT.S236701. PMID: 32158198. PMCID: PMC7049277.9. Seng Yue C, Ozdin D, Selber-Hnatiw S, Ducharme MP. 2019; Opportunities and challenges related to the implementation of model-based bioequivalence criteria. Clin Pharmacol Ther. 105:350–362. DOI: 10.1002/cpt.1270. PMID: 30375647.
Article10. Kim H, Han S, Cho YS, Yoon SK, Bae KS. 2018; Development of R packages: 'NonCompart' and 'ncar' for noncompartmental analysis (NCA). Transl Clin Pharmacol. 26:10–15. DOI: 10.12793/tcp.2018.26.1.10. PMID: 32055542. PMCID: PMC6989226.
Article11. U.S. Food and Drug Administration. 2013. Draft guidance for industry: bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA. U.S. Food and Drug Administration;Silver Spring: Available from: https://www.fdanews.com/ext/resources/files/12/12-05-13-ANDAGuidance.pdf. cited 2020 Nov 20.12. Heo YA, Holford N, Kim Y, Son M, Park K. 2016; Quantitative model for the blood pressure-lowering interaction of valsartan and amlodipine. Br J Clin Pharmacol. 82:1557–1567. DOI: 10.1111/bcp.13082. PMID: 27504853. PMCID: PMC5099563.
Article13. Wang T, Wang Y, Lin S, Fang L, Lou S, Zhao D, Zhu J, Yang Q, Wang Y. 2020; Evaluation of pharmacokinetics and safety with bioequivalence of Amlodipine in healthy Chinese volunteers: Bioequivalence Study Findings. J Clin Lab Anal. 34:e23228. DOI: 10.1002/jcla.23228. PMID: 32034814. PMCID: PMC7307347.
Article14. Maganti L, Panebianco DL, Maes AL. 2008; Evaluation of methods for estimating time to steady state with examples from phase 1 studies. AAPS J. 10:141–147. DOI: 10.1208/s12248-008-9014-y. PMID: 18446514. PMCID: PMC2751459.
Article15. Foster DM. Atkinson AJ Jr, Abernethy DR, Daniels CE, Dedrick RL, Markey SP, editors. 2007. Noncompartmental versus compartmental approaches to pharmacokinetic analysis. Principles of clinical pharmacology. Elsevier;Amsterdam: p. 89–105. DOI: 10.1016/B978-012369417-1/50048-1. PMID: 17593176. PMCID: PMC6932475.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Basic Principles of Drug Interaction
- Drug interaction: focusing on response surface models
- Drug-drug Interactions between Psychotropic Agents and Other Drugs in Physically Ill Patients: Experience of Consultation-liason in Korea University Hospital
- Drug-Drug Interactions: Mood Stabilizers and Anti-Anxiety Drugs
- How to design intravenous anesthetic dose regimens based on pharmacokinetics and pharmacodynamics principles