Korean J Physiol Pharmacol.
2021 Nov;25(6):517-523. 10.4196/kjpp.2021.25.6.517.
Effect of hydrogen-rich water on the lactic acid level in metformin-treated diabetic rats under hypoxia
- Affiliations
-
- 1Department of Pharmacy, the Sixth Medical Center of Chinese PLA General Hospital, Beijing 100048, China
- 2Department of Pharmacy, Medical Supplies Center of Chinese PLA General Hospital, Beijing 100853, China
- KMID: 2521477
- DOI: http://doi.org/10.4196/kjpp.2021.25.6.517
Abstract
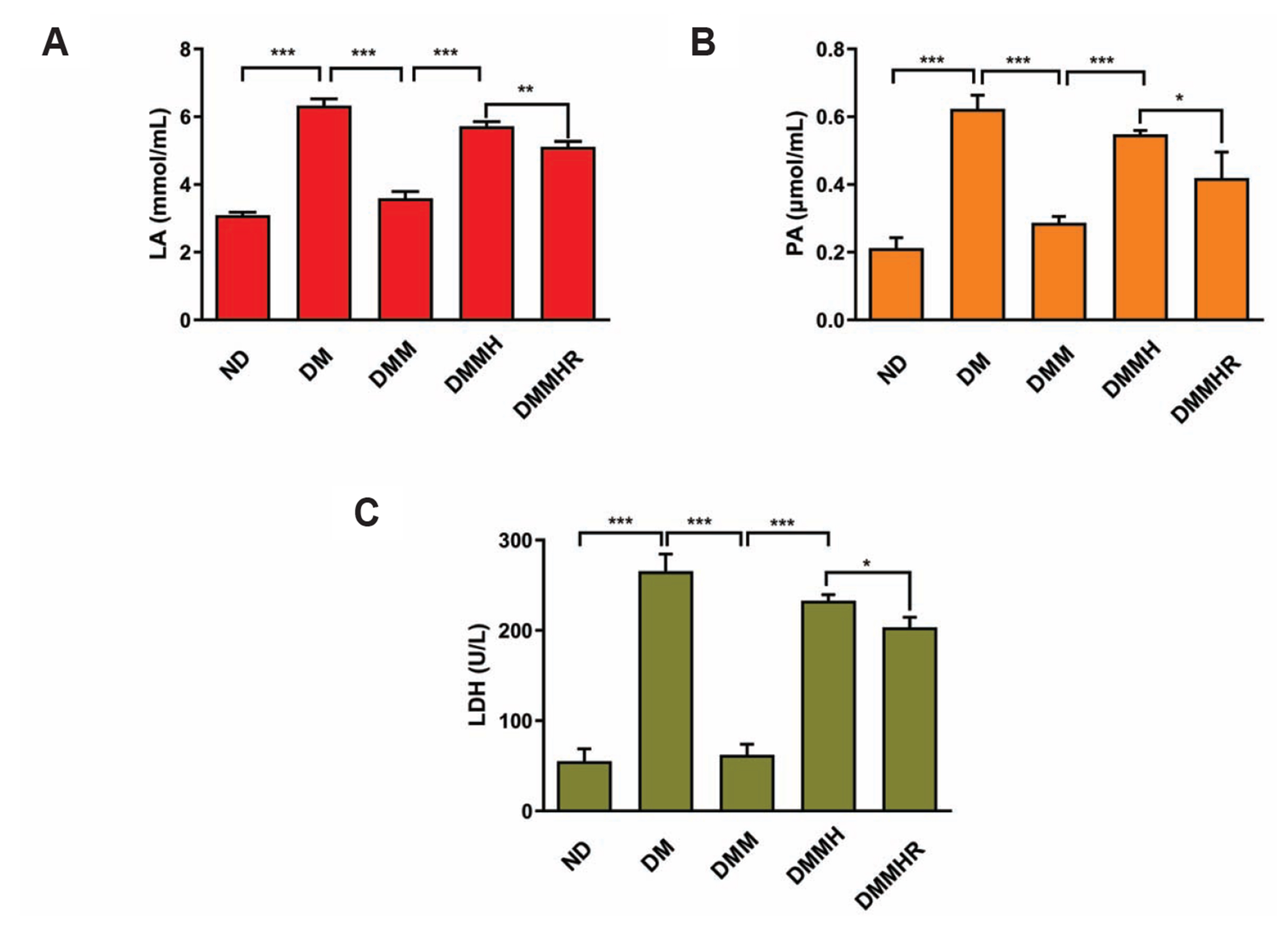
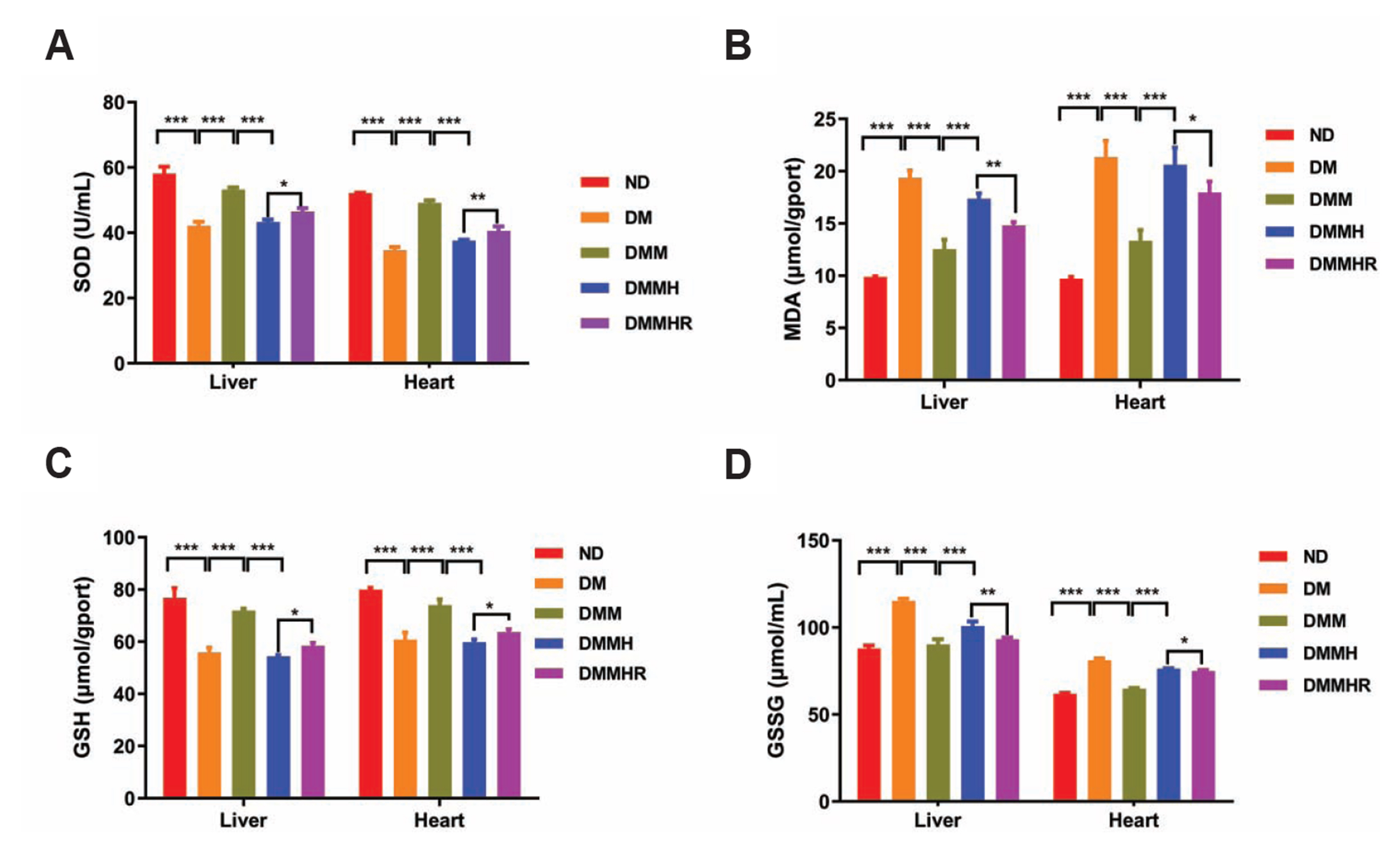
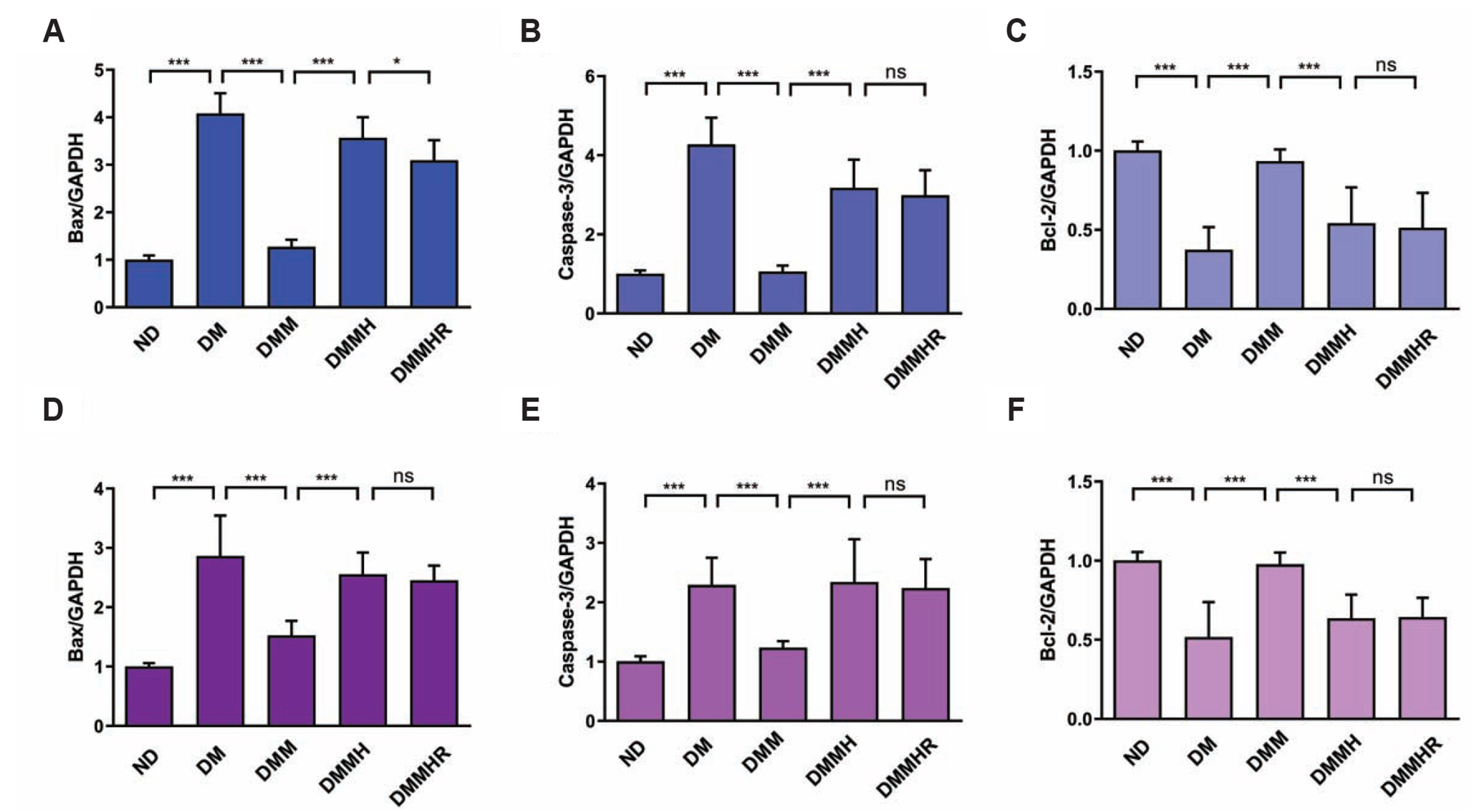
- The present study aims to investigate the impact of hydrogen-rich water on the lactic acid level in metformin-treated diabetic rats under hypoxia. Thirty Sprague–Dawley rats were randomly divided into five groups, including normal diet group, and diabetes model (DM) group, DM + metformin treatment (DMM) group, DMM + hypoxia treatment (DMMH) group and DMMH + hydrogenrich water (DMMHR) group. We found that the levels of lactic acid, pyruvate and lactate dehydrogenase were significantly lower in the blood of DMMHR group than DMMH group. Superoxide dismutase and glutathione levels in liver and heart were significantly higher in DMMH group after hydrogen-rich water treatment, while malondialdehyde and oxidized glutathione levels were decreased in DMMHR group when compared with DMMH group, which indicates that hydrogen-rich water could reduce oxidative stress. qPCR analysis demonstrated that that pro-apoptotic genes Bax/Caspase-3 were upregulated in DM group and metformin treatment suppressed their upregulation (DMM group). However, hypoxic condition reversed the effect of metformin on apoptotic gene expression, and hydrogen-rich water showed little effect on these genes under hypoxia. HE staining showed that hydrogen-rich water prevented myocardial fiber damages under hypoxia. In summary, we conclude that hydrogen-rich water could prevent lactate accumulation and reduce oxidant stress in diabetic rat model to prevent hypoxia-induced damages. It could be served as a potential agent for diabetes patients with metformin treatment to prevent lactic acidosis and reduce myocardial damages under hypoxic conditions.
Figure
Reference
-
1. Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. 2019; SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health. 16:2965. DOI: 10.3390/ijerph16162965. PMID: 31426529. PMCID: PMC6720282.
Article2. Zhang X, Yang L, Xu X, Tang F, Yi P, Qiu B, Hao Y. 2019; A review of fibroblast growth factor 21 in diabetic cardiomyopathy. Heart Fail Rev. 24:1005–1017. DOI: 10.1007/s10741-019-09809-x. PMID: 31175491.
Article3. An H, He L. 2016; Current understanding of metformin effect on the control of hyperglycemia in diabetes. J Endocrinol. 228:R97–R106. DOI: 10.1530/JOE-15-0447. PMID: 26743209. PMCID: PMC5077246.
Article4. Wróbel MP, Marek B, Kajdaniuk D, Rokicka D, Szymborska-Kajanek A, Strojek K. 2017; Metformin - a new old drug. Endokrynol Pol. 68:482–496. DOI: 10.5603/EP.2017.0050. PMID: 28819951.
Article5. Natali A, Ferrannini E. 2006; Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 49:434–441. DOI: 10.1007/s00125-006-0141-7. PMID: 16477438.
Article6. Ellul P, Delorme R, Cortese S. 2018; Metformin for weight gain associated with second-generation antipsychotics in children and adolescents: a systematic review and meta-analysis. CNS Drugs. 32:1103–1112. DOI: 10.1007/s40263-018-0571-z. PMID: 30238318.
Article7. Filippatos T, Tzavella E, Rizos C, Elisaf M, Liamis G. 2017; Acid-base and electrolyte disorders associated with the use of antidiabetic drugs. Expert Opin Drug Saf. 16:1121–1132. DOI: 10.1080/14740338.2017.1361400. PMID: 28748724.
Article8. DeFronzo R, Fleming GA, Chen K, Bicsak TA. 2016; Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 65:20–29. DOI: 10.1016/j.metabol.2015.10.014. PMID: 26773926.
Article9. Lalau JD. 2010; Lactic acidosis induced by metformin: incidence, management and prevention. Drug Saf. 33:727–740. DOI: 10.2165/11536790-000000000-00000. PMID: 20701406.10. Takaki A, Kawai D, Yamamoto K. 2013; Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 14:20704–20728. DOI: 10.3390/ijms141020704. PMID: 24132155. PMCID: PMC3821639.
Article11. Ohta S. 2011; Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des. 17:2241–2252. DOI: 10.2174/138161211797052664. PMID: 21736547. PMCID: PMC3257754.
Article12. Li S, Liao R, Sheng X, Luo X, Zhang X, Wen X, Zhou J, Peng K. 2019; Hydrogen gas in cancer treatment. Front Oncol. 9:696. DOI: 10.3389/fonc.2019.00696. PMID: 31448225. PMCID: PMC6691140.
Article13. Kamimura N, Nishimaki K, Ohsawa I, Ohta S. 2011; Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity (Silver Spring). 19:1396–1403. DOI: 10.1038/oby.2011.6. PMID: 21293445.
Article14. Aoki K, Nakao A, Adachi T, Matsui Y, Miyakawa S. 2012; Pilot study: effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. 2:12. DOI: 10.1186/2045-9912-2-12. PMID: 22520831. PMCID: PMC3395574.
Article15. Lenzen S. 2008; The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 51:216–226. DOI: 10.1007/s00125-007-0886-7. PMID: 18087688.
Article16. Scale T, Harvey JN. 2011; Diabetes, metformin and lactic acidosis. Clin Endocrinol (Oxf). 74:191–196. DOI: 10.1111/j.1365-2265.2010.03891.x. PMID: 21039727.
Article17. Bell DSH, Goncalves E. 2019; Heart failure in the patient with diabetes: epidemiology, aetiology, prognosis, therapy and the effect of glucose-lowering medications. Diabetes Obes Metab. 21:1277–1290. DOI: 10.1111/dom.13652. PMID: 30724013.
Article18. Fryer LG, Parbu-Patel A, Carling D. 2002; The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 277:25226–25232. DOI: 10.1074/jbc.M202489200. PMID: 11994296.
Article19. Hardie DG, Carling D. 1997; The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 246:259–273. DOI: 10.1111/j.1432-1033.1997.00259.x. PMID: 9208914.20. Han X, Yang J, Yang K, Zhao Z, Abendschein DR, Gross RW. 2007; Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry. 46:6417–6428. DOI: 10.1021/bi7004015. PMID: 17487985. PMCID: PMC2139909.
Article21. Brucculeri S, Urso C, Caimi G. 2013; [The role of lactate besides the lactic acidosis]. Clin Ter. 164:e223–e238. Italian. DOI: 10.7417/CT.2013.1572. PMID: 23868642.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Metformin-Induced Lactic Acidosis Successfully Treated by Hemodialysis
- Metformin induced acute pancreatitis and lactic acidosis in a patient on hemodialysis
- Effects of pH Differences on the Skin Moisturizing Capacity of Natural Moisturzing Factors
- A Case of Lactic Acidosis Associated with Prolonged Linezolid Therapy
- The peritoneal fluid lactic acid values in the stragulated intestinal obstruction in rats