Anat Cell Biol.
2021 Sep;54(3):375-386. 10.5115/acb.21.077.
Topical onion juice mitigates the morphological alterations of the cornea in the aged male rats
- Affiliations
-
- 1Department of Human Anatomy and Embryology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
- 2Department of Anatomy, College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Bahrain
- 3Department of Anatomy, Faculty of Medicine, The National University, Khartoum, Sudan
- KMID: 2521050
- DOI: http://doi.org/10.5115/acb.21.077
Abstract
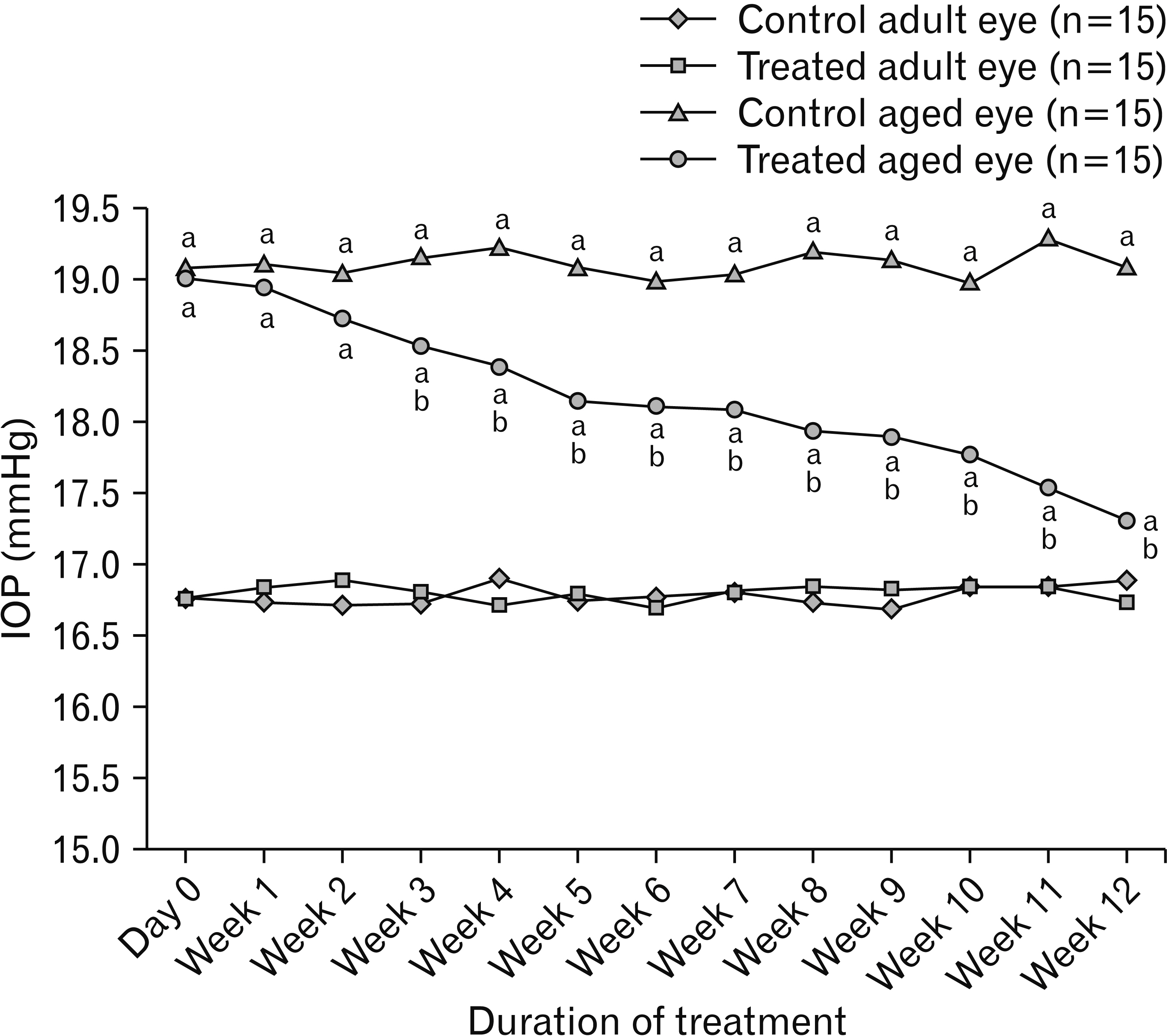
- Aging is associated with structural and functional changes of the cornea. Fresh onion juice contains phenolic compounds and flavonoids that may provide an anti-aging effect. The aim of this study was to assess the ability of the onion juice to ameliorate these aging changes. Rats were grouped as adult and aged groups. Rats of both groups received eye drops of diluted onion juice in their right eyes every 8 hours for 12 weeks, while the left ones were served as control eyes. The corneas of both eyes underwent histopathological, immunohistochemical and morphometric assessments, in addition to measuring their intraocular pressure (IOP). The aged group exhibited a significantly elevated IOP, decreased tear secretion, degenerated corneal epithelium and endothelium, surface erosions and stromal edema with irregular collagen fibers. Administration of onion juice led to lowering of IOP, significant increase in tearing, restoration of most of epithelial, endothelial and stromal integrity, and increased epithelial, keratocystic and endothelial cell densities. Immunohistochemically, the epithelium and endothelium revealed positive immune reactions for both epidermal growth factor receptor (EGFR) and paired box protein-6 (PAX6) in the control and onion-treated corneas of the adult group, while these immune reactions were negative in the untreated aged ones. Onion drops in aged corneas showed a positive immune reaction for EFGR and PAX6 involving the epithelial and endothelial layers. In conclusion, topical onion juice improves corneal aging changes through its direct effect, and indirectly through lowering IOP and enhancing tear secretion.
Figure
Reference
-
References
1. Greenwood MP, Greenwood M, Romanova EV, Mecawi AS, Paterson A, Sarenac O, Japundžić-Žigon N, Antunes-Rodrigues J, Paton JFR, Sweedler JV, Murphy D. 2018; The effects of aging on biosynthetic processes in the rat hypothalamic osmoregulatory neuroendocrine system. Neurobiol Aging. 65:178–91. DOI: 10.1016/j.neurobiolaging.2018.01.008. PMID: 29494864. PMCID: PMC5878011.
Article2. Lee B, Jung JH, Kim HS. 2012; Assessment of red onion on antioxidant activity in rat. Food Chem Toxicol. 50:3912–9. DOI: 10.1016/j.fct.2012.08.004. PMID: 22902824.
Article3. Mookherjee S, Bhattacharjee A, Sengupta M. 2015; The aging eye. J Ophthalmol. 2015:832326. DOI: 10.1155/2015/832326. PMID: 25878895. PMCID: PMC4387951.
Article4. Lin JB, Tsubota K, Apte RS. 2016; A glimpse at the aging eye. NPJ Aging Mech Dis. 2:16003. DOI: 10.1038/npjamd.2016.3. PMID: 28721262. PMCID: PMC5515005.
Article5. idhar MS Sr. 2018; Anatomy of cornea and ocular surface. Indian J Ophthalmol. 66:190–4. DOI: 10.4103/ijo.IJO_1005_17. PMID: 29676317. PMCID: PMC5939165.
Article6. Salvi SM, Akhtar S, Currie Z. 2006; Ageing changes in the eye. Postgrad Med J. 82:581–7. DOI: 10.1136/pgmj.2005.040857. PMID: 16954455. PMCID: PMC2585730.
Article7. Faragher RG, Mulholland B, Tuft SJ, Sandeman S, Khaw PT. 1997; Aging and the cornea. Br J Ophthalmol. 81:814–7. DOI: 10.1136/bjo.81.10.814. PMID: 9486017. PMCID: PMC1722015.
Article8. Gipson IK. 2013; Age-related changes and diseases of the ocular surface and cornea. Invest Ophthalmol Vis Sci. 54:ORSF48–53. DOI: 10.1167/iovs.13-12840. PMID: 24335068.
Article9. Obata H. 2006; Anatomy and histopathology of the human lacrimal gland. Cornea. 25(10 Suppl 1):S82–9. DOI: 10.1097/01.ico.0000247220.18295.d3. PMID: 17001201.
Article10. Aragona P, Giannaccare G, Mencucci R, Rubino P, Cantera E, Rolando M. 2021; Modern approach to the treatment of dry eye, a complex multifactorial disease: a P.I.C.A.S.S.O. board review. Br J Ophthalmol. 105:446–53. DOI: 10.1136/bjophthalmol-2019-315747. PMID: 32703782. PMCID: PMC8005804.11. Mete R, Oran M, Topcu B, Oznur M, Seber ES, Gedikbasi A, Yetisyigit T. 2016; Protective effects of onion (Allium cepa) extract against doxorubicin-induced hepatotoxicity in rats. Toxicol Ind Health. 32:551–7. DOI: 10.1177/0748233713504807. PMID: 24193056.
Article12. Lee SU, Lee JH, Choi SH, Lee JS, Ohnisi-Kameyama M, Kozukue N, Levin CE, Friedman M. 2008; Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J Agric Food Chem. 56:8541–8. DOI: 10.1021/jf801009p. PMID: 18759442.
Article13. Nan L, Huang J, Li Y, Cui C, Peng G, Xie B, Liu H, Liu Y. 2019; Study on the extraction and antioxidant properties of flavonoids from onion. AIP Conf Proc. 2079:020005. DOI: 10.1063/1.5092383.
Article14. Wilson EA, Demmig-Adams B. 2007; Antioxidant, anti-inflammatory, and antimicrobial properties of garlic and onions. Nutr Food Sci. 37:178–83. DOI: 10.1108/00346650710749071.
Article15. Upadhyay RK. 2016; Nutraceutical, pharmaceutical and therapeutic uses of Allium cepa: a review. Int J Green Pharm. 10(Suppl):S46–64.16. Eady CC, Kamoi T, Kato M, Porter NG, Davis S, Shaw M, Kamoi A, Imai S. 2008; Silencing onion lachrymatory factor synthase causes a significant change in the sulfur secondary metabolite profile. Plant Physiol. 147:2096–106. DOI: 10.1104/pp.108.123273. PMID: 18583530. PMCID: PMC2492635.
Article17. Dartt DA, Willcox MD. 2013; Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp Eye Res. 117:1–3. DOI: 10.1016/j.exer.2013.10.008. PMID: 24280033. PMCID: PMC4225770.
Article18. Kim S, Park YW, Lee E, Park SW, Park S, Noh H, Kim JW, Seong JK, Seo K. 2016; Effect of onion extract on corneal haze suppression after air assisted lamellar keratectomy. J Vet Med Sci. 78:419–25. DOI: 10.1292/jvms.15-0455. PMID: 26607134. PMCID: PMC4829509.
Article19. Javadzadeh A, Ghorbanihaghjo A, Bonyadi S, Rashidi MR, Mesgari M, Rashtchizadeh N, Argani H. 2009; Preventive effect of onion juice on selenite-induced experimental cataract. Indian J Ophthalmol. 57:185–9. DOI: 10.4103/0301-4738.49391. PMID: 19384011. PMCID: PMC2683439.
Article20. Nejabat M, Salehi A, Noorani Azad P, Ashraf MJ. 2014; Effects of onion juice on the normal flora of eyelids and conjunctiva in an animal model. Jundishapur J Microbiol. 7:e9678. DOI: 10.5812/jjm.9678. PMID: 25147716. PMCID: PMC4138639.
Article21. Owji N, Khalili MR, Khademi B, Shirvani M, Sadati MS. 2020; Comparison of the effectiveness of onion extract, topical steroid, and petrolatum emollient in cosmetic appearance of upper blepharoplasty scar. J Curr Ophthalmol. 32:408–13. DOI: 10.4103/JOCO.JOCO_39_20. PMID: 33553845. PMCID: PMC7861112.22. El-Demerdash FM, Yousef MI, El-Naga NI. 2005; Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 43:57–63. DOI: 10.1016/j.fct.2004.08.012. PMID: 15582196.
Article23. Yu H, Zhong H, Chen J, Sun J, Huang P, Xu X, Huang S, Zhong Y. 2020; Efficacy, drug sensitivity, and safety of a chronic ocular hypertension rat model established using a single intracameral injection of hydrogel into the anterior chamber. Med Sci Monit. 26:e925852. DOI: 10.12659/MSM.925852. PMID: 32997651. PMCID: PMC7534505.
Article24. Dias AC, Módulo CM, Jorge AG, Braz AM, Jordão AA Jr, Filho RB, de Paula JS, Rocha EM. 2007; Influence of thyroid hormone on thyroid hormone receptor beta-1 expression and lacrimal gland and ocular surface morphology. Invest Ophthalmol Vis Sci. 48:3038–42. DOI: 10.1167/iovs.06-1309. PMID: 17591870.25. Bancroft JD, Gamble M. 2008. Theory and practice of histological techniques. 6th ed. Churchill Livingstone Elsevier;Philadelphia: p. 72.26. Hwang HB, Oh TH, Kim HS. 2013; Effect of ethanol-treated mid-peripheral epithelium on corneal wound healing in rabbits. BMC Ophthalmol. 13:27. DOI: 10.1186/1471-2415-13-27. PMID: 23822645. PMCID: PMC3703275.
Article27. Lei H, Liu H, Ding Y, Ge L. 2014; Immunohistochemical localization of Pax6 in the developing tooth germ of mice. J Mol Histol. 45:373–9. DOI: 10.1007/s10735-014-9564-5. PMID: 24477661.
Article28. Burgess D, Zhang Y, Siefker E, Vaca R, Kuracha MR, Reneker L, Overbeek PA, Govindarajan V. 2010; Activated Ras alters lens and corneal development through induction of distinct downstream targets. BMC Dev Biol. 10:13. DOI: 10.1186/1471-213X-10-13. PMID: 20105280. PMCID: PMC2828409.
Article29. Wang G, Chen P, Wang Y, Wang Y, Reinach PS, Xue Y, Liu Z, Li C. 2020; Onion epithelial membrane scaffolds transfer corneal epithelial layers in reconstruction surgery. Adv Healthc Mater. 9:e2000469. DOI: 10.1002/adhm.202000469. PMID: 32548957.
Article30. Kumar S, Modgil S, Bammidi S, Minhas G, Shri R, Kaushik S, Singh V, Anand A. 2020; Allium cepa exerts neuroprotective effect on retinal ganglion cells of pterygopalatine artery (PPA) ligated mice. J Ayurveda Integr Med. 11:489–94. DOI: 10.1016/j.jaim.2019.08.002. PMID: 32088091. PMCID: PMC7772493.
Article31. Pradeep SR, inivasan K Sr. 2018; Ameliorative influence of dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) on eye lens abnormalities via modulation of crystallin proteins and polyol pathway in experimental diabetes. Curr Eye Res. 43:1108–18. DOI: 10.1080/02713683.2018.1484146. PMID: 29856678.
Article32. Inomata T, Mashaghi A, Hong J, Nakao T, Dana R. 2017; Scaling and maintenance of corneal thickness during aging. PLoS One. e0185694. DOI: 10.1371/journal.pone.0185694. PMID: 28985226. PMCID: PMC5630165.
Article33. El-Sayyad HIH, El-Mansi AA, Guida MS, Mohammed EA. 2015; Markers characterizing corneal damage during aging of rat. J Adv Chem. 11:3532–9. DOI: 10.24297/jac.v11i5.4471.
Article34. Halawa AM. 2011; Age-associated changes in the cornea, lens and retina of the albino rat eye: a histological and immuno-histochemical study. Egyptian J Anat. 34:1–13. DOI: 10.21608/ejana.2011.3649.
Article35. Baroody RA, Bito LZ, DeRousseau CJ, Kaufman PL. 1987; Ocular development and aging. 1. Corneal endothelial changes in cats and in free-ranging and caged rhesus monkeys. Exp Eye Res. 45:607–22. DOI: 10.1016/S0014-4835(87)80070-2. PMID: 3428387.
Article36. Tananuvat N, Khumchoo N. 2020; Corneal thickness and endothelial morphology in normal Thai eyes. BMC Ophthalmol. 20:167. DOI: 10.1186/s12886-020-01385-1. PMID: 32345246. PMCID: PMC7187506.
Article37. Yang Y, Hong J, Deng SX, Xu J. 2014; Age-related changes in human corneal epithelial thickness measured with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 55:5032–8. DOI: 10.1167/iovs.13-13831. PMID: 25052994.
Article38. Lagali N, Germundsson J, Fagerholm P. 2009; The role of Bowman's layer in corneal regeneration after phototherapeutic keratectomy: a prospective study using in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 50:4192–8. DOI: 10.1167/iovs.09-3781. PMID: 19407024.39. Zhou Q, Wang Y, Yang L, Wang Y, Chen P, Wang Y, Dong X, Xie L. 2008; Histone deacetylase inhibitors blocked activation and caused senescence of corneal stromal cells. Mol Vis. 14:2556–65. PMID: 19122829. PMCID: PMC2613076.40. Patel S, McLaren J, Hodge D, Bourne W. 2001; Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 42:333–9. PMID: 11157863.41. Niederer RL, Perumal D, Sherwin T, McGhee CN. 2007; Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 91:1165–9. DOI: 10.1136/bjo.2006.112656. PMID: 17389741. PMCID: PMC1954900.42. Gambato C, Longhin E, Catania AG, Lazzarini D, Parrozzani R, Midena E. 2015; Aging and corneal layers: an in vivo corneal confocal microscopy study. Graefes Arch Clin Exp Ophthalmol. 253:267–75. DOI: 10.1007/s00417-014-2812-2. PMID: 25311652.43. Jun AS, Chakravarti S, Edelhauser HF, Kimos M. 2006; Aging changes of mouse corneal endothelium and Descemet's membrane. Exp Eye Res. 83:890–6. DOI: 10.1016/j.exer.2006.03.025. PMID: 16777092.
Article44. Johnson DH, Bourne WM, Campbell RJ. 1982; The ultrastructure of Descemet's membrane. I. Changes with age in normal corneas. Arch Ophthalmol. 100:1942–7. DOI: 10.1001/archopht.1982.01030040922011. PMID: 7150061.45. Xiao X, Wang Y, Gong H, Chen P, Xie L. 2009; Molecular evidence of senescence in corneal endothelial cells of senescence-accelerated mice. Mol Vis. 15:747–61. PMID: 19381346. PMCID: PMC2669445.46. Cheng H, Jacobs PM, McPherson K, Noble MJ. 1985; Precision of cell density estimates and endothelial cell loss with age. Arch Ophthalmol. 103:1478–81. DOI: 10.1001/archopht.1985.01050100054017. PMID: 4051849.
Article47. Jorge J, Queirós A, Blasco TF, González-Méijome JM. Peixoto de Matos SC. 2010; Age-related changes of corneal endothelium in normal eyes with a non-contact specular microscope. J Emmetropia. 1:132–9.48. Zhang H, Qin X, Cao X, Zhang D, Li L. 2017; Age-related variations of rabbit corneal geometrical and clinical biomechanical parameters. Biomed Res Int. 2017:3684971. DOI: 10.1155/2017/3684971. PMID: 29104870. PMCID: PMC5574220.
Article49. Dubicanac M, Joly M, Strüve J, Nolte I, Mestre-Francés N, Verdier JM, Zimmermann E. 2018; Intraocular pressure in the smallest primate aging model: the gray mouse lemur. Vet Ophthalmol. 21:319–27. DOI: 10.1111/vop.12434. PMID: 27624923.
Article50. Sandhas E, Merle R, Eule JC. 2018; Consider the eye in preventive healthcare- ocular findings, intraocular pressure and Schirmer tear test in ageing cats. J Feline Med Surg. 20:1063–71. DOI: 10.1177/1098612X17742528. PMID: 29172875.51. Melamed S, Ben-Sira I, Ben-Shaul Y. 1980; Corneal endothelial changes under induced intraocular pressure elevation: a scanning and transmission electron microscopic study in rabbits. Br J Ophthalmol. 64:164–9. DOI: 10.1136/bjo.64.3.164. PMID: 7387948. PMCID: PMC1039380.
Article52. Janson BJ, Alward WL, Kwon YH, Bettis DI, Fingert JH, Provencher LM, Goins KM, Wagoner MD, Greiner MA. 2018; Glaucoma-associated corneal endothelial cell damage: a review. Surv Ophthalmol. 63:500–6. DOI: 10.1016/j.survophthal.2017.11.002. PMID: 29146208.
Article53. Yu ZY, Wu L, Qu B. 2019; Changes in corneal endothelial cell density in patients with primary open-angle glaucoma. World J Clin Cases. 7:1978–85. DOI: 10.12998/wjcc.v7.i15.1978. PMID: 31423429. PMCID: PMC6695540.
Article54. Beesk N, Perner H, Schwarz D, George E, Kroh LW, Rohn S. 2010; Distribution of quercetin-3,4'-O-diglucoside, quercetin-4'-O-monoglucoside, and quercetin in different parts of the onion bulb (Allium cepa L.) influenced by genotype. Food Chem. 122:566–71. DOI: 10.1016/j.foodchem.2010.03.011.
Article55. Boots AW, Wilms LC, Swennen EL, Kleinjans JC, Bast A, Haenen GR. 2008; In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition. 24:703–10. DOI: 10.1016/j.nut.2008.03.023. PMID: 18549926.56. Ozgen S, Kilinc OK, Selamoglu Z. 2016; Antioxidant activity of quercetin: a mechanistic review. Turkish J Agric Food Sci Technol. 4:1134–8. DOI: 10.24925/turjaf.v4i12.1134-1138.1069.
Article57. Zhao L, Wang H, Du X. 2021; The therapeutic use of quercetin in ophthalmology: recent applications. Biomed Pharmacother. 137:111371. DOI: 10.1016/j.biopha.2021.111371. PMID: 33561647.
Article58. Song X, Wang Y, Gao L. 2020; Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. J Mol Model. 26:133. DOI: 10.1007/s00894-020-04356-x. PMID: 32399900.
Article59. Xiao X, Shi D, Liu L, Wang J, Xie X, Kang T, Deng W. 2011; Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS One. 6:e22934. DOI: 10.1371/journal.pone.0022934. PMID: 21857970. PMCID: PMC3152552.
Article60. Kliuiev GO, Kolomiichuk SG. 2017; The research of the effect of eye drops containing quercetin for regeneration of the cornea in the experiment. J Tradit Med Clin Natur. 6:222. DOI: 10.4172/2573-4555.1000222.61. Maugeri G, D'Amico AG, Castrogiovanni P, Saccone S, Federico C, Reibaldi M, Russo A, Bonfiglio V, Avitabile T, Longo A, D'Agata V. 2019; PACAP through EGFR transactivation preserves human corneal endothelial integrity. J Cell Biochem. 120:10097–105. DOI: 10.1002/jcb.28293. PMID: 30548314.
Article62. Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. 2000; Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 41:1346–55. PMID: 10798649.63. Davis J, Duncan MK, Robison WG Jr, Piatigorsky J. 2003; Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci. 116(Pt 11):2157–67. DOI: 10.1242/jcs.00441. PMID: 12692153.
Article64. Mort RL, Bentley AJ, Martin FL, Collinson JM, Douvaras P, Hill RE, Morley SD, Fullwood NJ, West JD. 2011; Effects of aberrant Pax6 gene dosage on mouse corneal pathophysiology and corneal epithelial homeostasis. PLoS One. 6:e28895. DOI: 10.1371/journal.pone.0028895. PMID: 22220198. PMCID: PMC3248408.
Article65. Chen HB, Yamabayashi S, Ou B, Tanaka Y, Ohno S, Tsukahara S. 1997; Structure and composition of rat precorneal tear film. A study by an in vivo cryofixation. Invest Ophthalmol Vis Sci. 38:381–7. PMID: 9040471.66. Cramer CS, Mandal S, Sharma S, Nourbakhsh SS, Goldman I, Guzman I. 2021; Recent advances in onion genetic improvement. Agronomy. 11:482. DOI: 10.3390/agronomy11030482.
Article67. Wilson SE, Lloyd SA, Kennedy RH. 1991; Epidermal growth factor messenger RNA production in human lacrimal gland. Cornea. 10:519–24. DOI: 10.1097/00003226-199111000-00010. PMID: 1723672.
Article68. Kang SS, Li T, Xu D, Reinach PS, Lu L. 2000; Inhibitory effect of PGE2 on EGF-induced MAP kinase activity and rabbit corneal epithelial proliferation. Invest Ophthalmol Vis Sci. 41:2164–9. PMID: 10892858.69. Wilson SE, Liu JJ, Mohan RR. 1999; Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 18:293–309. DOI: 10.1016/S1350-9462(98)00017-2. PMID: 10192515.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of Vegetable Juice Fermented with Lactobacillus plantarum MKHA15 and Leuconostoc mesenteroids MKSR
- Two Cases of Bleeding Tendency in Patients with Long-term Taking Onion-juice
- Alterations in the blood glucose, serum lipids and renal oxidative stress in diabetic rats by supplementation of onion (Allium cepa. Linn)
- Concentration in the Cornea After Topical Administration of 1% Clotrimazole in Rabbits
- Antioxidant Effects on various solvent extracts from Onion Peel and Onion Flesh