Nutr Res Pract.
2021 Oct;15(5):591-603. 10.4162/nrp.2021.15.5.591.
Hesperetin suppresses LPS/high glucose-induced inflammatory responses via TLR/MyD88/NF-κB signaling pathways in THP-1 cells
- Affiliations
-
- 1Department of Food and Nutrition, Chonnam National University, Gwangju 61186, Korea
- 2Department of Education, Graduate School of Education, Chonnam National University, Gwangju 61186, Korea
- KMID: 2520661
- DOI: http://doi.org/10.4162/nrp.2021.15.5.591
Abstract
- BACKGROUND/OBJECTIVES
Unregulated inflammatory responses caused by hyperglycemia may induce diabetes complications. Hesperetin, a bioflavonoid, is a glycoside in citrus fruits and is known to have antioxidant and anticarcinogenic properties. However, the effect of inflammation on the diabetic environment has not been reported to date. In this study, we investigated the effect of hesperetin on proinflammatory cytokine secretion and its underlying mechanistic regulation in THP-1 macrophages with co-treatment LPS and hyperglycemic conditions.
MATERIALS/METHODS
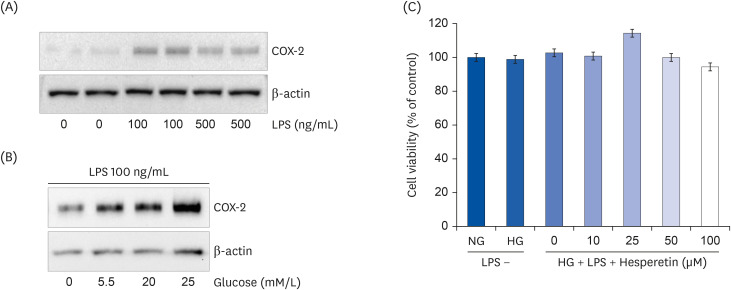
THP-1 cells differentiated by PMA (1 μM) were cultured for 48 h in the presence or absence of hesperetin under normoglycemic (5.5 mM/L glucose) or hyperglycemic (25 mM/L glucose) conditions and then treated with LPS (100 ng/mL) for 6 h before harvesting. Inflammation-related proteins and mRNA levels were evaluated by enzyme-linked immunosorbent assay, western blot, and quantitative polymerase chain reaction analyses.
RESULTS
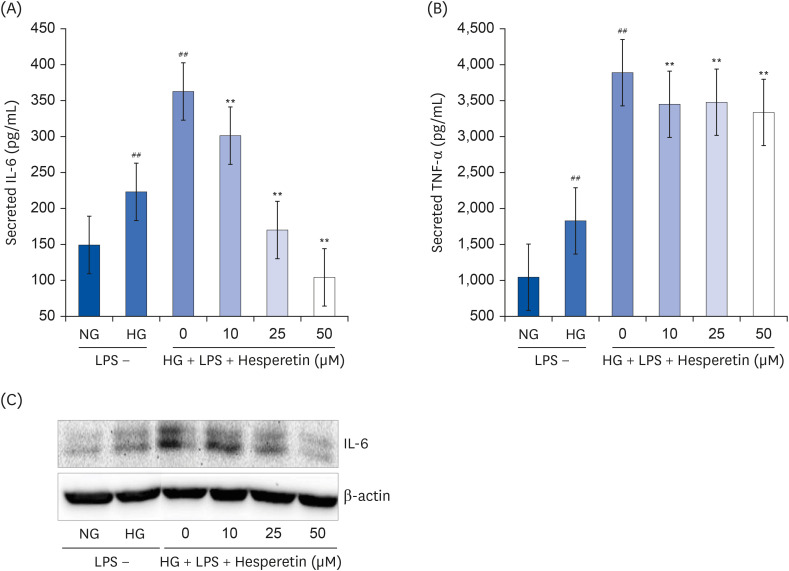
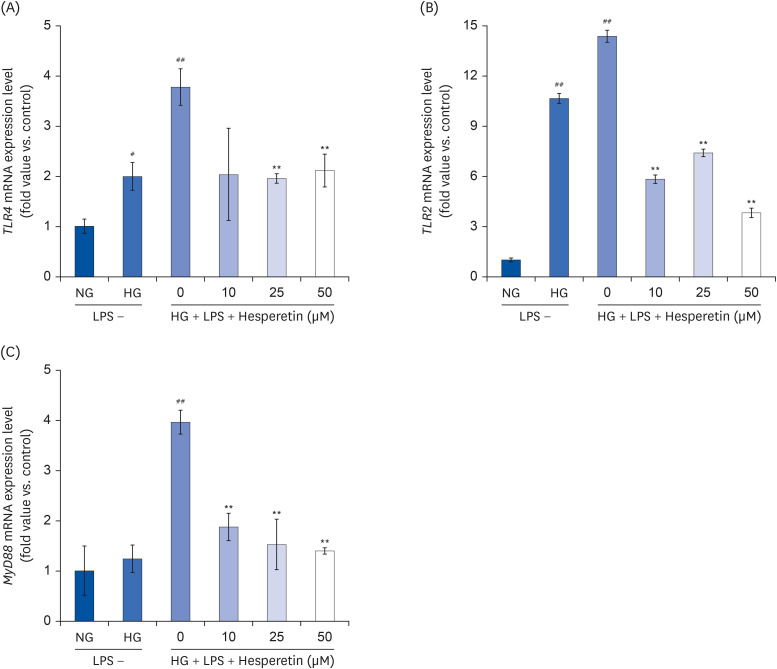
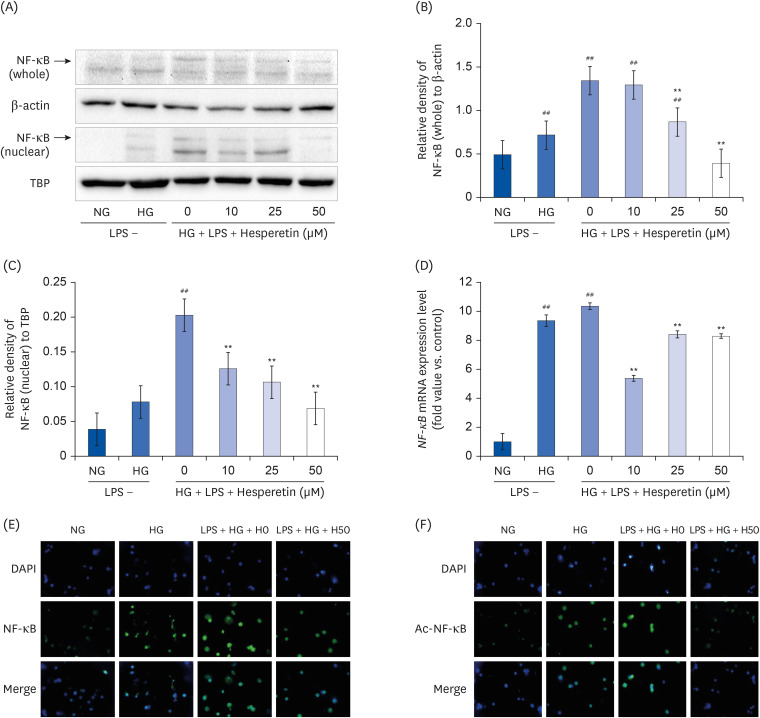
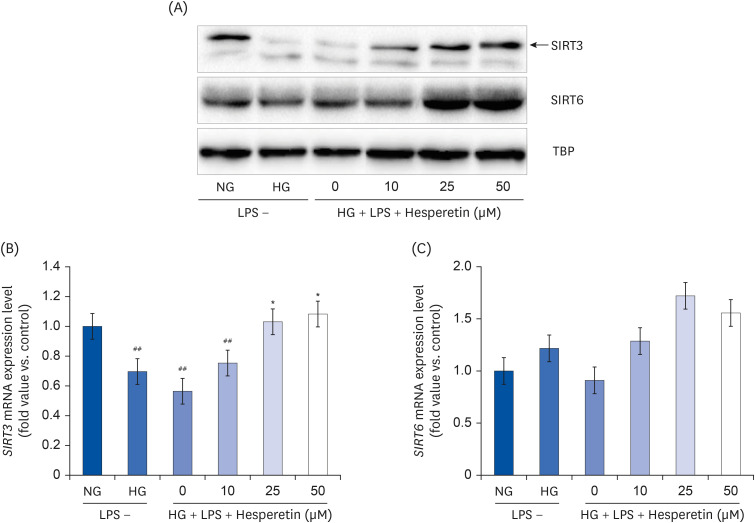
Hesperetin (0–100 μM, 48 h) treatment did not affect cell viability. The tumor necrosis factor-α and interleukin-6 levels increased in cells co-treated with LPS under hyperglycemic conditions compared to normoglycemic conditions, and these increases were decreased by hesperetin treatment. The TLR2/4 and MyD88 activity levels increased in cells co-treated with LPS under hyperglycemic conditions compared to normoglycemic conditions; however, hesperetin treatment inhibited the TLR2/4 and MyD88 activity increases. In addition, nuclear factor-κB (NF-κB) and Acetyl-NF-κB levels increased in response to treatment with LPS under hyperglycemic conditions compared to normoglycemic conditions, but those levels were decreased when treated with hesperetin. SIRT3 and SIRT6 expressions were increased by hesperetin treatment.
CONCLUSIONS
Our results suggest that hesperetin may be a potential agent for suppressing inflammation in diabetes.
Keyword
Figure
Cited by 1 articles
-
L -Methionine inhibits 4-hydroxy-2-nonenal accumulation and suppresses inflammation in growing rats
Zhengxuan Wang, Mingcai Liang, Hui Li, Bingxiao Liu, Lin Yang
Nutr Res Pract. 2022;16(6):729-744. doi: 10.4162/nrp.2022.16.6.729.
Reference
-
1. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000; 355:773–778. PMID: 10711923.
Article2. Urakami T, Kubota S, Nitadori Y, Harada K, Owada M, Kitagawa T. Annual incidence and clinical characteristics of type 2 diabetes in children as detected by urine glucose screening in the Tokyo metropolitan area. Diabetes Care. 2005; 28:1876–1881. PMID: 16043726.
Article3. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019; 157:107843. PMID: 31518657.4. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011; 121:2111–2117. PMID: 21633179.
Article5. Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev. 2001; 22:36–52. PMID: 11159815.
Article6. Shanmugam N, Kim YS, Lanting L, Natarajan R. Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem. 2003; 278:34834–34844. PMID: 12837757.
Article7. de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005; 25:904–914. PMID: 15731497.8. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005; 11:191–198. PMID: 15685170.9. Yang SM, Kim SY, Lee KY, Kim YS, Nam MS, Park IB. Inflammatory markers are associated with microvascular complications in type 2 diabetes. J Korean Diabetes Assoc. 2007; 31:472–479.
Article10. Dasu MR, Ramirez S, Isseroff RR. Toll-like receptors and diabetes: a therapeutic perspective. Clin Sci (Lond). 2012; 122:203–214. PMID: 22070434.
Article11. Rhee SH, Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000; 275:34035–34040. PMID: 10952994.12. Liang H, Hussey SE, Sanchez-Avila A, Tantiwong P, Musi N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS One. 2013; 8:e63983. PMID: 23704966.
Article13. Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013; 45:e66–66. PMID: 24310172.
Article14. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019; 20:20.
Article15. Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010; 5:253–295. PMID: 20078221.
Article16. Bosch-Presegué L, Vaquero A. The dual role of sirtuins in cancer. Genes Cancer. 2011; 2:648–662. PMID: 21941620.17. Ma Y, Chen H, He X, Nie H, Hong Y, Sheng C, Wang Q, Xia W, Ying W. NAD+ metabolism and NAD(+)-dependent enzymes: promising therapeutic targets for neurological diseases. Curr Drug Targets. 2012; 13:222–229. PMID: 22204321.
Article18. Roth M, Chen WY. Sorting out functions of sirtuins in cancer. Oncogene. 2014; 33:1609–1620. PMID: 23604120.
Article19. Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999; 260:273–279. PMID: 10381378.
Article20. Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005; 16:4623–4635. PMID: 16079181.
Article21. Wang CH, Wei YH. Roles of mitochondrial sirtuins in mitochondrial function, redox homeostasis, insulin resistance and type 2 diabetes. Int J Mol Sci. 2020; 21:5266.
Article22. Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009; 136:62–74. PMID: 19135889.23. Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015; 29:323–331. PMID: 25394264.
Article24. Aranganathan S, Nalini N. Efficacy of the potential chemopreventive agent, hesperetin (citrus flavanone), on 1,2-dimethylhydrazine induced colon carcinogenesis. Food Chem Toxicol. 2009; 47:2594–2600. PMID: 19632289.
Article25. Morin B, Nichols LA, Zalasky KM, Davis JW, Manthey JA, Holland LJ. The citrus flavonoids hesperetin and nobiletin differentially regulate low density lipoprotein receptor gene transcription in HepG2 liver cells. J Nutr. 2008; 138:1274–1281. PMID: 18567747.
Article26. Kumar B, Gupta SK, Srinivasan BP, Nag TC, Srivastava S, Saxena R, Jha KA. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc Res. 2013; 87:65–74. PMID: 23376836.
Article27. Jialal I, Kaur H. The role of toll-like receptors in diabetes-induced inflammation: implications for vascular complications. Curr Diab Rep. 2012; 12:172–179.
Article28. Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008; 4:46–54. PMID: 18220695.29. Nishikawa T, Araki E. Investigation of a novel mechanism of diabetic complications: impacts of mitochondrial reactive oxygen species. Rinsho Byori. 2008; 56:712–719. PMID: 18800628.30. Jain SK, Kannan K, Lim G, Matthews-Greer J, McVie R, Bocchini JA Jr. Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003; 26:2139–2143. PMID: 12832326.
Article31. Jain SK, Kannan K, Lim G, McVie R, Bocchini JA Jr. Hyperketonemia increases tumor necrosis factor-alpha secretion in cultured U937 monocytes and Type 1 diabetic patients and is apparently mediated by oxidative stress and cAMP deficiency. Diabetes. 2002; 51:2287–2293. PMID: 12086962.32. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001; 280:E745–51. PMID: 11287357.
Article33. Yun JM, Jialal I, Devaraj S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J Nutr Biochem. 2011; 22:450–458. PMID: 20655188.
Article34. Matulewicz N, Karczewska-Kupczewska M. Insulin resistance and chronic inflammation. Postepy Hig Med Dosw. 2016; 70:1245–1258.35. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. 2020; 126:1549–1564. PMID: 32437299.
Article36. Hossain M, Faruque MO, Kabir G, Hassan N, Sikdar D, Nahar Q, Ali L. Association of serum TNF-α and IL-6 with insulin secretion and insulin resistance in IFG and IGT subjects in a Bangladeshi population. Int J Diabetes Mellit. 2010; 2:165–168.
Article37. Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999; 353:1649–1652. PMID: 10335783.
Article38. Xie C, Kang J, Ferguson ME, Nagarajan S, Badger TM, Wu X. Blueberries reduce pro-inflammatory cytokine TNF-α and IL-6 production in mouse macrophages by inhibiting NF-κB activation and the MAPK pathway. Mol Nutr Food Res. 2011; 55:1587–1591. PMID: 21887820.
Article39. Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J Biol Chem. 2000; 275:17728–17739. PMID: 10837498.40. Choi RY, Ham JR, Lee MK. Esculetin prevents non-alcoholic fatty liver in diabetic mice fed high-fat diet. Chem Biol Interact. 2016; 260:13–21. PMID: 27769711.
Article41. Lee H, Yang SJ. In vitro and in vivo effects of piceatannol and resveratrol on glucose control and TLR4-NF-κB pathway. J Korean Soc Food Sci Nutr. 2017; 46:267–272.42. Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005; 175:3339–3346. PMID: 16116226.
Article43. Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010; 91:940–949. PMID: 20200256.
Article44. Hua KF, Wang SH, Dong WC, Lin CY, Ho CL, Wu TH. High glucose increases nitric oxide generation in lipopolysaccharide-activated macrophages by enhancing activity of protein kinase C-α/δ and NF-κB. Inflamm Res. 2012; 61:1107–1116. PMID: 22706318.
Article45. Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998; 128:2334–2340. PMID: 9868178.
Article46. Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999; 126:673–680. PMID: 10188978.47. Chan MM, Mattiacci JA, Hwang HS, Shah A, Fong D. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem Pharmacol. 2000; 60:1539–1548. PMID: 11020457.48. Huynh FK, Hershberger KA, Hirschey MD. Targeting sirtuins for the treatment of diabetes. Diabetes Manag (Lond). 2013; 3:245–257. PMID: 25067957.
Article49. Elliott P, Walpole S, Morelli L, Lambert P, Lunsmann W, Westphal C, Lavu S.Resveratrol/SRT-501. Drugs Fut. 2009; 34:291–295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sodium butyrate inhibits high glucose-induced inflammation by controlling the acetylation of NF-κB p65 in human monocytes
- Meconopsis quintuplinervia Regel Improves Cutibacterium acnes-Induced Inflammatory Responses in a Mouse Ear Edema Model and Suppresses Pro-Inflammatory Chemokine Production via the MAPK and NF-κB Pathways in RAW264.7 Cells
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- Beauvericin, a cyclic peptide, inhibits inflammatory responses in macrophages by inhibiting the NF-κB pathway
- MIR210HG Aggravates Sepsis-Induced Inflammatory Response of Proximal Tubular Epithelial Cell via the NF-κB Signaling Pathway