Ann Pediatr Endocrinol Metab.
2021 Sep;26(3):171-177. 10.6065/apem.2040134.067.
Short-term efficacy of 1-month and 3-month gonadotropin-releasing hormone agonist depots in girls with central precocious puberty
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, Korea
- 2Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2520509
- DOI: http://doi.org/10.6065/apem.2040134.067
Abstract
- Purpose
Gonadotropin-releasing hormone agonist (GnRHa) has been the mainstay of central precocious puberty (CPP) treatment for decades, but few reports have compared the efficacy of 1-month and 3-month depot GnRHa formulations. This study investigates the short-term efficacy of 1-month and 3-month GnRHa depots in girls with CPP.
Methods
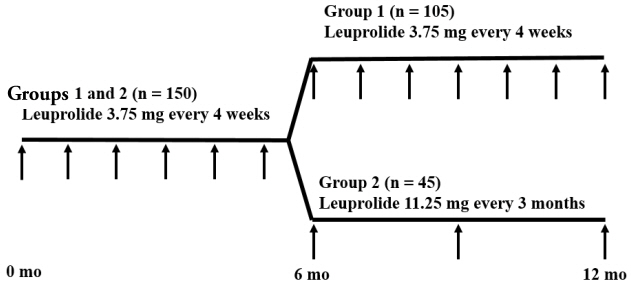
Overall, 150 girls with CPP were included in a retrospective review of medical records. Subjects in group 1 (n=105) were treated with 1-month GnRHa depots for ≥12 months, and those in group 2 (n=45) were treated with 1-month GnRHa depots for 6 months followed by 3-month GnRHa depots for ≥6 months. Anthropometric and biochemical data were compared between the groups at 3-time points (after 0, 6, and 12 months of GnRHa treatment).
Results
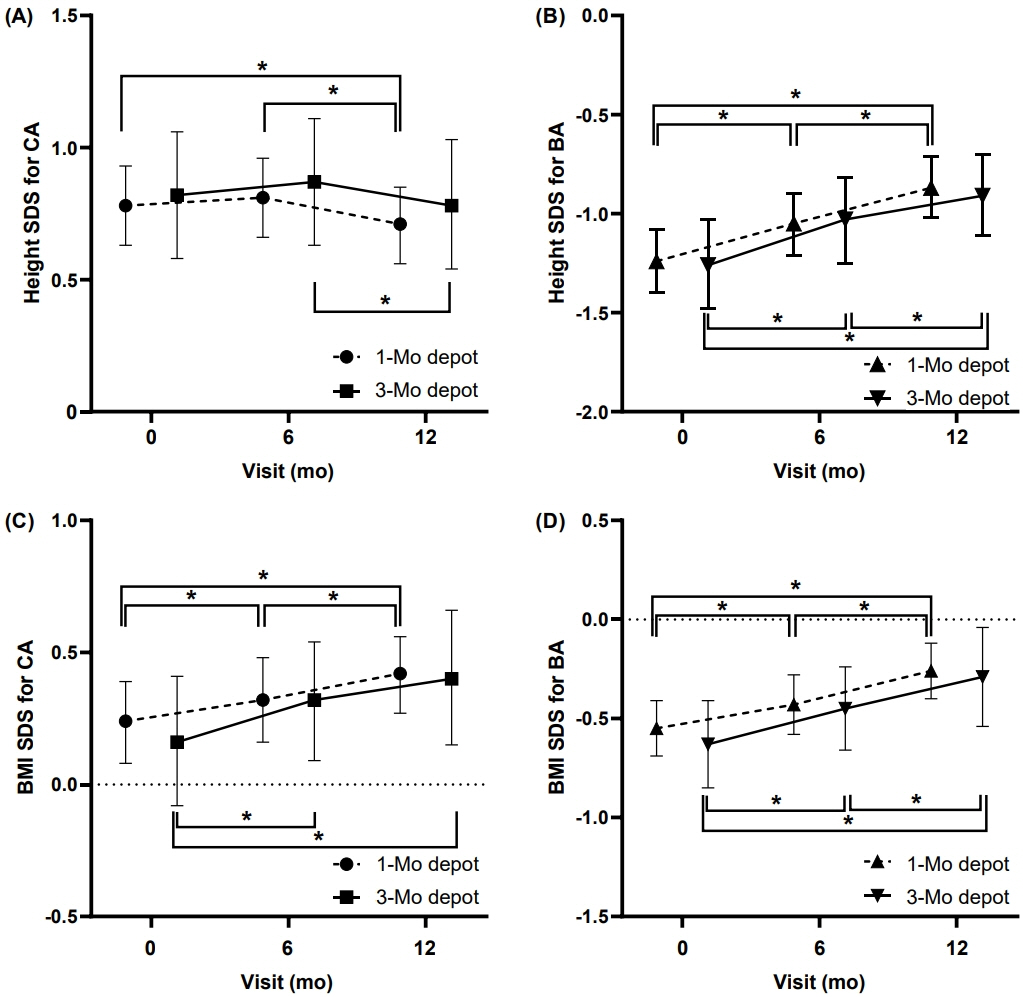
Demographic and clinical characteristics did not differ between the groups at baseline or after 6 months of GnRHa treatment. After 12 months of GnRHa treatment, patients in the both groups showed no difference in bone age (BA), chronological age (CA), BA–CA difference, height standard deviation score (SDS) for CA and BA, or body mass index SDS for CA and BA. The sexual maturity rate of the breast was prepubertal at 12 months in most of subjects. GnRH-stimulated luteinizing hormone (LH) level was suppressed during GnRHa treatment in both groups at 6 and 12 months, although the LH level in group 2 was higher than that in group 1.
Conclusion
Treating CPP with a 3-month GnRHa depot showed short-term efficacy comparable to that with a 1-month depot in anthropometric parameters and pubertal suppression.
Figure
Cited by 1 articles
-
Long-term efficacy of a triptorelin 3-month depot in girls with central precocious puberty
Kyu Hyun Park, Si-Hwa Gwag, Yu Jin Kim, Lindsey Yoojin Chung, Eungu Kang, Hyo-Kyoung Nam, Young-Jun Rhie, Kee-Hyoung Lee
Ann Pediatr Endocrinol Metab. 2024;29(3):161-166. doi: 10.6065/apem.2346132.066.
Reference
-
References
1. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003; 24:668–93.
Article2. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016; 4:265–74.
Article3. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, Group E-LGACC, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009; 123:e752–62.
Article4. Parker KL, Lee PA. Depot leuprolide acetate for treatment of pre c o cious pub er ty. J C lin E ndo crinol Me t ab. 1989; 69:689–91.5. Mul D, Hughes IA. The use of GnRH agonists in precocious puberty. Eur J Endocrinol. 2008; 159 Suppl 1:S3–8.
Article6. Partsch CJ, Heger S, Sippell WG. Management and outcome of central precocious puberty. Clin Endocrinol (Oxf ). 2002; 56:129–48.
Article7. Bertelloni S, Baroncelli GI, Ferdeghini M, MenchiniFabris F, Saggese G. Final height, gonadal function and bone mineral density of adolescent males with central precocious puberty after therapy with gonadotropin-releasing hormone analogues. Eur J Pediatr. 2000; 159:369–74.
Article8. Badaru A, Wilson DM, Bachrach LK, Fechner P, Gandrud LM, Durham E, et al. Sequential comparisons of onemonth and three-month depot leuprolide regimens in central precocious puberty. J Clin Endocrinol Metab. 2006; 91:1862–7.
Article9. Isaac H, Patel L, Meyer S, Hall CM, Cusick C, Price DA, et al. Efficacy of a monthly compared to 3-monthly depot GnRH analogue (goserelin) in the treatment of children with central precocious puberty. Horm Res. 2007; 68:157–63.
Article10. Fuld K, Chi C, Neely EK. A randomized trial of 1- and 3-month depot leuprolide doses in the treatment of central precocious puberty. J Pediatr. 2011; 159:982–7. e1.
Article11. Lee PA, Klein K, Mauras N, Neely EK, Bloch CA, Larsen L, et al. Efficacy and safety of leuprolide acetate 3-month depot 11.25 milligrams or 30 milligrams for the treatment of central precocious puberty. J Clin Endocr Metab. 2012; 97:1572–80.
Article12. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.
Article13. Greulich WW, Pyle SI. R adiologic atlas of skeletal development of the hand and wrist. 2nd ed. Redwood City (CA): Stanford University Press;1959.14. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969; 44:291–303.
Article15. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952; 40:423–41.
Article16. Kim YJ, Lee HS, Lee YJ, Lim JS, Kim SY, Kim EY, et al. Multicenter clinical trial of leuprolide acetate depot (Luphere depot 3.75 mg) for efficacy and safety in girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2013; 18:173–8.
Article17. Carel JC, Lahlou N, Jaramillo O, Montauban V, Teinturier C, Colle M, et al. Treatment of central precocious puberty by subcutaneous injections of leuprorelin 3-month depot (11.25 mg). J Clin Endocrinol Metab. 2002; 87:4111–6.
Article18. Lee PA, Klein K, Mauras N, Lev-Vaisler T, Bacher P. 36-month treatment experience of two doses of leuprolide acetate 3-month depot for children with central precocious puberty. J Clin Endocrinol Metab. 2014; 99:3153–9.
Article19. Durand A, Tauber M, Patel B, Dutailly P. Meta-analysis of paediatric patients with central precocious puberty treated with intramuscular triptorelin 11.25 mg 3-month prolonged-release formulation. Horm Res Paediatr. 2017; 87:224–32.
Article20. Bertelloni S, Baroncelli GI, Sorrentino MC, Perri G, Saggese G. Effect of central precocious puberty and gonadotropinreleasing hormone analogue treatment on peak bone mass and final height in females. Eur J Pediatr. 1998; 157:363–7.
Article21. Bertelloni S, Massart F, Einaudi S, Wasniewska M, Miccoli M, Baroncelli GI. Central precocious puberty: adult height in girls treated with quarterly or monthly gonadotropinreleasing hormone analog triptorelin. Horm Res Paediatr. 2015; 84:396–400.
Article22. Kunz GJ, Sherman TI, Klein KO. Luteinizing hormone (LH) and estradiol suppression and growth in girls with central precocious puberty: is more suppression better? Are pre-injection LH levels useful in monitoring treatment? J Pediatr Endocrinol Metab. 2007; 20:1189–98.
Article23. Lanes R, Soros A, Jakubowicz S. Accelerated versus slowly progressive forms of puberty in girls with precocious and early puberty. Gonadotropin suppressive effect and final height obtained with two different analogs. J Pediatr Endocrinol Metab. 2004; 17:759–66.
Article24. Traggiai C, Perucchin PP, Zerbini K, Gastaldi R, De Biasio P, Lorini R. Outcome after depot gonadotrophin-releasing hormone agonist treatment for central precocious puberty: effects on body mass index and final height. Eur J Endocrinol. 2005; 153:463–4.
Article25. Vuralli D, Ozon ZA, Gonc EN, Alikasifoglu A, Kandemir N. Long-term effects of GnRH agonist treatment on body mass index in girls with idiopathic central precocious puberty. J Pediatr Endocrinol Metab. 2020; 33:99–105.
Article26. Censani M, Feuer A, Orton S, Askin G, Vogiatzi M. Changes in body mass index in children on gonadotropin-releasing hormone agonist therapy with precocious puberty, early puberty or short stature. J Pediatr Endocrinol Metab. 2019; 32:1065–70.
Article27. Paterson WF, McNeill E, Young D, Donaldson MDC. Auxological outcome and time to menarche following longacting goserelin therapy in girls with central precocious or early puberty. Clin Endocrinol. 2004; 61:626–34.
Article28. Yoon JW, Park HA, Lee J, Kim JH. The influence of gonadotropin-releasing hormone agonists on anthropometric change in girls with central precocious puberty. Korean J Pediatr. 2017; 60:395–402.
Article29. Bangalore Krishna K, Fuqua JS, Rogol AD, Klein KO, Popovic J, Houk CP, et al. Use of gonadotropin-releasing hormone analogs in children: update by an international consortium. Horm Res Paediatr. 2019; 91:357–72.
Article30. Tanaka T, Niimi H, Matsuo N, Fujieda K, Tachibana K, Ohyama K, et al. Results of long-term follow-up after treatment of central precocious puberty with leuprorelin acetate: evaluation of effectiveness of treatment and recovery of gonadal function. The TAP-144-SR Japanese Study Group on Central Precocious Puberty. J Clin Endocrinol Metab. 2005; 90:1371–6.
Article31. Carel JC, Lahlou N, Guazzarotti L, Joubert-Collin M, Roger M, Colle M, et al. Treatment of central precocious puberty with depot leuprorelin. French Leuprorelin Trial Group. Eur J Endocrinol. 1995; 132:699–704.32. Choi JH. Proper dosage and duration of GnRH agonist treatment in central precocious puberty. J Korean Soc Pediatr Endocrinol. 2006; 11:8–14.33. Jin HY, Choi JH, Yoo HW. Evaluation of efficacy of GnRH agonist on predicted adult height (PAH) in patients with central precocious puberty using two different dosages. J Korean Soc Pediatr Endocrinol. 2010; 15:120–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Indication of Gonadotropin-Releasing Hormone Agonist: Precocious Puberty or Advanced Puberty?
- Serum IGF-1 and IGFBP-3 Levels in Central Precocious Puberty Girls Treated with Gonadotropin Releasing Hormone Agonist (GnRHa)
- Efficacy of Triptorelin 3-Month Depot Compared to 1-Month Depot for the Treatment of Korean Girls with Central Precocious Puberty in Single Tertiary Center
- Auxological Effects of Gonadotropin-releasing Hormone Agonist Treatment for Central Precocious Puberty
- A comparative study of the puberty suppression effect of gonadotropin-releasing hormone agonist in precocious or early puberty girls