J Korean Med Sci.
2021 Aug;36(34):e219. 10.3346/jkms.2021.36.e219.
Efficacy of Triptorelin 3-Month Depot Compared to 1-Month Depot for the Treatment of Korean Girls with Central Precocious Puberty in Single Tertiary Center
- Affiliations
-
- 1Department of Pediatrics, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
- 2Department of Pediatrics, Korea University College of Medicine, Seoul, Korea
- KMID: 2519595
- DOI: http://doi.org/10.3346/jkms.2021.36.e219
Abstract
- Background
Triptorelin depot is largely used to treat central precocious puberty (CPP) in children, and a 3-month depot has been introduced. However, data about the 3-month gonadotropin-releasing hormone use for treatment of CPP in Korean girls are not available. This study was conducted to compare the efficacy of a triptorelin 11.25 mg 3-month depot with that of a 3.75 mg 1-month depot in suppressing pubertal development for the treatment of CPP.
Methods
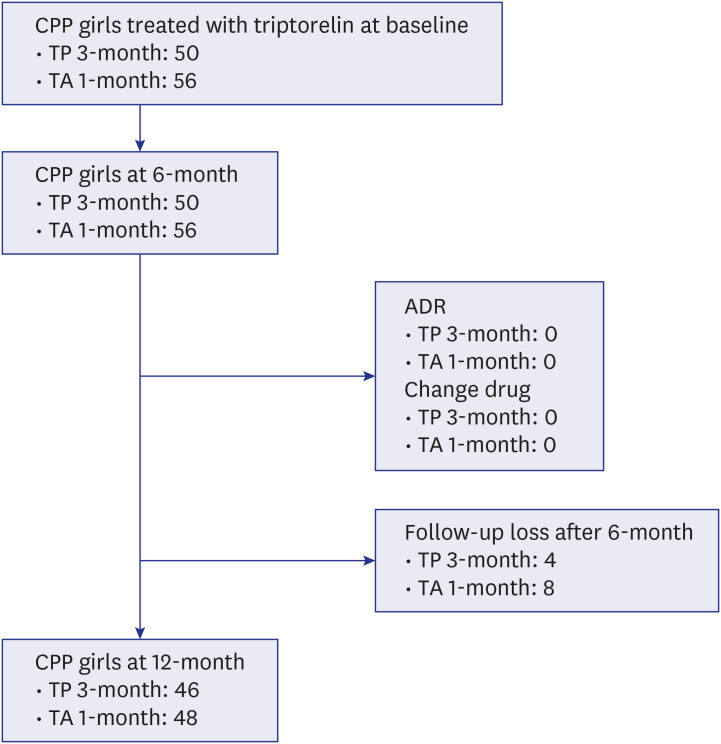
A retrospective study, including 106 girls with CPP treated with triptorelin, was conducted. Fifty patients were treated with a triptorelin 3-month depot, and 56 were treated with a triptorelin 1-month depot. Serum luteinizing hormone (LH), follicle-stimulating hormone, and estradiol levels were analysed every 6 months after the visit. The height and bone age of each patient was evaluated at the beginning of treatment, after 6 months, and one year after therapy.
Results
The baseline characteristics of the girls treated with a 3-month depot were similar to those of the girls treated with a 1-month depot. A suppressed levels of LH to the triptorelin injection (serum LH < 2.5 IU/L) at 6 months was seen in 90.0% and 98.2% of the girls treated with the 3-month and 1-month depots, respectively (P = 0.160). After 1 year of treatment, a suppressed levels of LH was seen in 93.5% and 100% of the girls treated with the 3-month and 1-month depots, respectively (P = 0.226). Height velocity showed no significant difference between the two groups. Degree of bone age advancement decreased from 1.22 ± 0.07 and 1.22 ± 0.08 years at baseline (P = 0.914) to 1.16 ± 0.07 and 1.17 ± 0.08 in the girls treated with the 3-month and 1-month depots after 1 year, respectively (P = 0.481).
Conclusion
This study showed that the efficacy of long-acting triptorelin 3-month was comparable to 1-month depot regarding hormonal suppression and inhibition of bone maturation. The triptorelin 11.25 mg 3-month depot is an effective treatment for girls with CPP.
Keyword
Figure
Cited by 1 articles
-
Long-term efficacy of a triptorelin 3-month depot in girls with central precocious puberty
Kyu Hyun Park, Si-Hwa Gwag, Yu Jin Kim, Lindsey Yoojin Chung, Eungu Kang, Hyo-Kyoung Nam, Young-Jun Rhie, Kee-Hyoung Lee
Ann Pediatr Endocrinol Metab. 2024;29(3):161-166. doi: 10.6065/apem.2346132.066.
Reference
-
1. Nebesio TD, Eugster EA. Current concepts in normal and abnormal puberty. Curr Probl Pediatr Adolesc Health Care. 2007; 37(2):50–72. PMID: 17223057.
Article2. Nathan BM, Palmert MR. Regulation and disorders of pubertal timing. Endocrinol Metab Clin North Am. 2005; 34(3):617–641. PMID: 16085163.
Article3. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, Antoniazzi F, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009; 123(4):e752–e762. PMID: 19332438.
Article4. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, Antoniazzi F, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009; 123(4):e752–e762. PMID: 19332438.
Article5. Kim YJ, Lee HS, Lee YJ, Lim JS, Kim SY, Kim EY, et al. Multicenter clinical trial of leuprolide acetate depot (Luphere depot 3.75 mg) for efficacy and safety in girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2013; 18(4):173–178. PMID: 24904873.
Article6. Müller J, Juul A, Andersson AM, Sehested A, Skakkebaek NE. Hormonal changes during GnRH analogue therapy in children with central precocious puberty. J Pediatr Endocrinol Metab. 2000; 13(Suppl 1):739–746. PMID: 10969916.
Article7. Baek JW, Nam HK, Jin D, Oh YJ, Rhie YJ, Lee KH. Age of menarche and near adult height after long-term gonadotropin-releasing hormone agonist treatment in girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2014; 19(1):27–31. PMID: 24926460.
Article8. Padula AM, Macmillan KL. Oestradiol-17beta responsiveness, plasma LH profiles, pituitary LH and FSH concentrations in long-term ovariectomised Holstein cows at 24 h, 48 h and 21 days following treatment with an absorbable GnRH agonist implant. Anim Reprod Sci. 2005; 85(1-2):27–39. PMID: 15556306.9. Lahlou N, Carel JC, Chaussain JL, Roger M. Pharmacokinetics and pharmacodynamics of GnRH agonists: clinical implications in pediatrics. J Pediatr Endocrinol Metab. 2000; 13(Suppl 1):723–737. PMID: 10969915.
Article10. Chiocca E, Dati E, Baroncelli GI, Cassio A, Wasniewska M, Galluzzi F, et al. Central precocious puberty: treatment with triptorelin 11.25 mg. Sci World J. 2012; 2012:583751.11. Carel JC, Blumberg J, Seymour C, Adamsbaum C, Lahlou N. Triptorelin 3-month CPP Study Group. Three-month sustained-release triptorelin (11.25 mg) in the treatment of central precocious puberty. Eur J Endocrinol. 2006; 154(1):119–124. PMID: 16382000.
Article12. Martínez-Aguayo A, Hernández MI, Beas F, Iñiguez G, Avila A, Sovino H, et al. Treatment of central precocious puberty with triptorelin 11.25 mg depot formulation. J Pediatr Endocrinol Metab. 2006; 19(8):963–970. PMID: 16995580.
Article13. Badaru A, Wilson DM, Bachrach LK, Fechner P, Gandrud LM, Durham E, et al. Sequential comparisons of one-month and three-month depot leuprolide regimens in central precocious puberty. J Clin Endocrinol Metab. 2006; 91(5):1862–1867. PMID: 16449344.
Article14. Fuld K, Chi C, Neely EK. A randomized trial of 1- and 3-month depot leuprolide doses in the treatment of central precocious puberty. J Pediatr. 2011; 159(6):982–987.e1. PMID: 21798557.
Article15. Neely EK, Wilson DM, Lee PA, Stene M, Hintz RL. Spontaneous serum gonadotropin concentrations in the evaluation of precocious puberty. J Pediatr. 1995; 127(1):47–52. PMID: 7608810.
Article16. Ab Rahim SN, Omar J, Tuan Ismail TS. Gonadotropin-releasing hormone stimulation test and diagnostic cutoff in precocious puberty: a mini review. Ann Pediatr Endocrinol Metab. 2020; 25(3):152–155. PMID: 32871650.
Article17. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist: based on the brush foundation study of human growth and development initiated by T. Wingate Todd, M.B., Ch. B., F.R.C.S. JAMA. 1950; 143(12):1124.18. Kim YM, Choi JH, Lee BH, Yoo HW. Efficacy of a single luteinizing hormone measurement after GnRH agonist administration for therapeutic monitoring of girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2012; 17(3):153–159.
Article19. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean national growth charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61(5):135–149. PMID: 29853938.
Article20. Tanner JM. Growth at Adolescence. Oxford, London: Blackwell;1962.21. Bertelloni S, Arrigo T, Wasniewska M, Galluzzi F, Einaudi S, Cappa M, et al. Central precocious puberty: short-term comparative data of treatment with monthly or long-acting three months depot triptorelin. J Pediatr Endocrinol Metab. 2007; 20:297–305.22. Bertelloni S, Massart F, Einaudi S, Wasniewska M, Miccoli M, Baroncelli GI. Central precocious puberty: adult height in girls treated with quarterly or monthly gonadotropin-releasing hormone analog triptorelin. Horm Res Paediatr. 2015; 84(6):396–400. PMID: 26528763.
Article23. Heo S, Lee YS, Yu J. Basal serum luteinizing hormone value as the screening biomarker in female central precocious puberty. Ann Pediatr Endocrinol Metab. 2019; 24(3):164–171. PMID: 31607109.
Article24. Houk CP, Kunselman AR, Lee PA. The diagnostic value of a brief GnRH analogue stimulation test in girls with central precocious puberty: a single 30-minute post-stimulation LH sample is adequate. J Pediatr Endocrinol Metab. 2008; 21(12):1113–1118. PMID: 19189683.
Article25. Lee PA, Luce M, Bacher P. Monitoring treatment of central precocious puberty using basal luteinizing hormone levels and practical considerations for dosing with a 3-month leuprolide acetate formulation. J Pediatr Endocrinol Metab. 2016; 29(11):1249–1257. PMID: 27740929.
Article26. Demirbilek H, Alikasifoglu A, Gonc NE, Ozon A, Kandemir N. Assessment of gonadotrophin suppression in girls treated with GnRH analogue for central precocious puberty; validity of single luteinizing hormone measurement after leuprolide acetate injection. Clin Endocrinol (Oxf). 2012; 76(1):126–130. PMID: 21790701.
Article27. Brito VN, Latronico AC, Arnhold IJ, Mendonca BB. A single luteinizing hormone determination 2 hours after depot leuprolide is useful for therapy monitoring of gonadotropin-dependent precocious puberty in girls. J Clin Endocrinol Metab. 2004; 89(9):4338–4342. PMID: 15356030.
Article28. Lucaccioni L, McNeilly J, Mason A, Giacomozzi C, Kyriakou A, Shaikh MG, et al. The measurement of urinary gonadotropins for assessment and management of pubertal disorder. Hormones (Athens). 2016; 15(3):377–384. PMID: 27838606.
Article29. Poomthavorn P, Khlairit P, Mahachoklertwattana P. Subcutaneous gonadotropin-releasing hormone agonist (triptorelin) test for diagnosing precocious puberty. Horm Res. 2009; 72(2):114–119. PMID: 19690429.
Article30. Lee PA. Laboratory monitoring of children with precocious puberty. Arch Pediatr Adolesc Med. 1994; 148(4):369–376. PMID: 8148936.
Article31. Eckert KL, Wilson DM, Bachrach LK, Anhalt H, Habiby RL, Olney RC, et al. A single-sample, subcutaneous gonadotropin-releasing hormone test for central precocious puberty. Pediatrics. 1996; 97(4):517–519. PMID: 8632938.
Article32. Neely EK, Hintz RL, Wilson DM, Lee PA, Gautier T, Argente J, et al. Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr. 1995; 127(1):40–46. PMID: 7608809.
Article33. Martínez-Aguayo A, Hernández MI, Beas F, Iñiguez G, Avila A, Sovino H, et al. Treatment of central precocious puberty with triptorelin 11.25 mg depot formulation. J Pediatr Endocrinol Metab. 2006; 19(8):963–970. PMID: 16995580.
Article34. Zenaty D, Blumberg J, Liyanage N, Jacqz-Aigrain E, Lahlou N, Carel JC. A 6-month trial of the efficacy and safety of triptorelin pamoate (11.25 mg) every 3 months in children with precocious puberty: a retrospective comparison with triptorelin acetate. Horm Res Paediatr. 2016; 86(3):188–195. PMID: 27603324.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term efficacy of a triptorelin 3-month depot in girls with central precocious puberty
- An 8-year-old girl with drug hypersensitivity to first triptorelin acetate administration
- Short-term efficacy of 1-month and 3-month gonadotropin-releasing hormone agonist depots in girls with central precocious puberty
- Multicenter clinical trial of leuprolide acetate depot (Luphere depot 3.75 mg) for efficacy and safety in girls with central precocious puberty
- Sterile Abscess Formation Associated with Two Different Forms of Gonadotropin-Releasing Hormone Agonist in Central Precocious Puberty