J Korean Med Sci.
2021 Sep;36(37):e239. 10.3346/jkms.2021.36.e239.
Effects of Advanced Glycation End Products on Differentiation and Function of Osteoblasts and Osteoclasts
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Kyung Hee University Hospital, Seoul, Korea
- 2Division of Cardiology, Asan Medical Center, Seoul, Korea
- 3Department of Endocrinology and Metabolism, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea
- KMID: 2520365
- DOI: http://doi.org/10.3346/jkms.2021.36.e239
Abstract
- Background
Risk of fragility fractures increases in patients with diabetes mellitus, independent of bone mineral density. In the present study, the effects of advanced glycation end products (AGEs) on differentiation and function of osteoblasts and osteoclasts were investigated.
Methods
AGEs and 25 mM glucose were administered to marrow-derived macrophages and MCT3T3-E1 cells. The effects of AGEs on osteoclast differentiation was investigated using tartrate-resistant acid phosphatase (TRAP) assay. The effects of AGEs on osteoblast differentiation was investigated using alkaline phosphatase (ALP) activity and bone nodule formation assays. Expression of osteoclast-specific and osteoblast-specific genes and effects on cell signaling pathways associated with cell differentiation were analyzed using reverse transcription polymerase chain reaction and western blotting.
Results
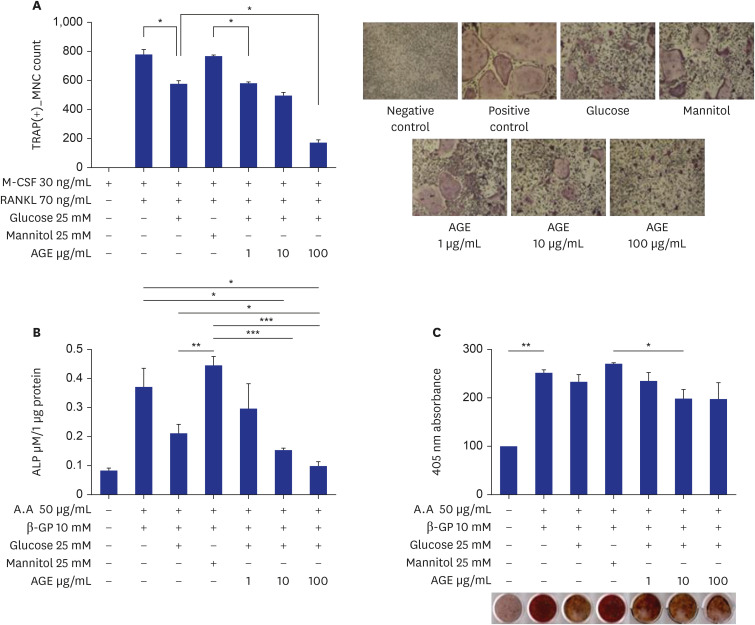
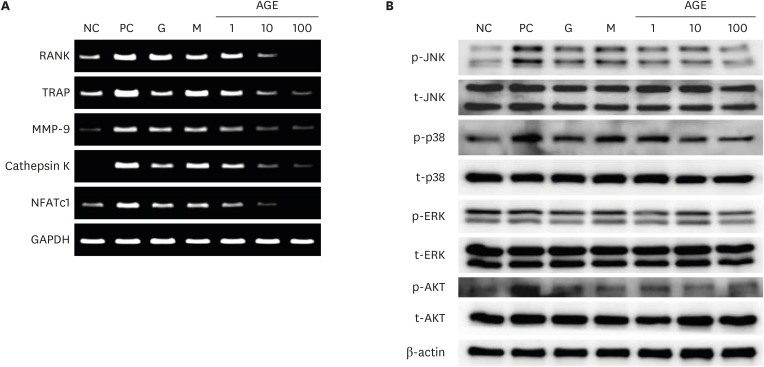
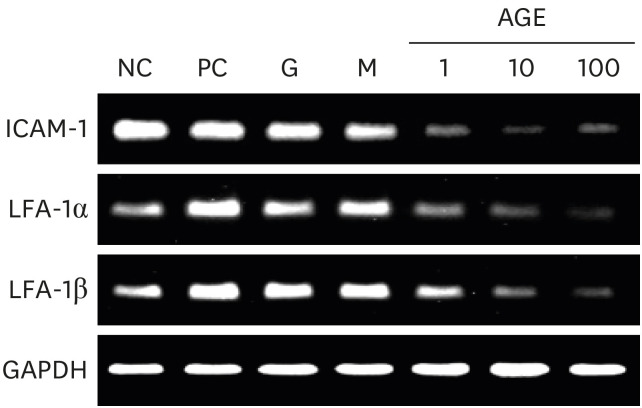
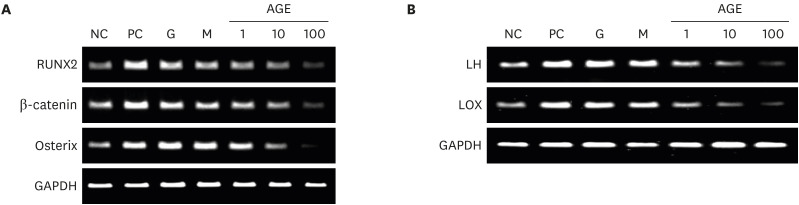
AGEs significantly decreased TRAP-positive multinucleated cell formation in receptor activator of nuclear factor-κB ligand-induced marrow-derived macrophages in a dose-dependent manner. AGEs suppressed the expression of osteoclast-specific genes, JNK, p38, AKT, intercellular adhesion molecule 1, and lymphocyte function-associated antigen 1 in marrow-derived macrophages. AGEs decreased ALP activity and showed a tendency to decrease bone nodule formation in MC3T3-E1 cells. AGEs suppressed the expression of osteoblast-specific genes, lysyl hydroxylase and lysyl oxidase in MC3T3-E1 cells.
Conclusion
AGEs suppressed differentiation and function of osteoclasts and osteoblasts, and collagen cross-linking activity. It suggests that AGE may induce bone fragility through low bone turnover and deterioration of bone quality.
Figure
Reference
-
1. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007; 18(4):427–444. PMID: 17068657.
Article2. Wang J, You W, Jing Z, Wang R, Fu Z, Wang Y. Increased risk of vertebral fracture in patients with diabetes: a meta-analysis of cohort studies. Int Orthop. 2016; 40(6):1299–1307. PMID: 27029481.
Article3. Hough FS, Pierroz DD, Cooper C, Ferrari SL, Bone IC. IOF CSA Bone and Diabetes Working Group. Mechanisms in endocrinology: mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016; 174(4):R127–R138. PMID: 26537861.
Article4. Brown SJ, Handsaker JC, Bowling FL, Boulton AJ, Reeves ND. Diabetic peripheral neuropathy compromises balance during daily activities. Diabetes Care. 2015; 38(6):1116–1122. PMID: 25765355.
Article5. Vavanikunnel J, Charlier S, Becker C, Schneider C, Jick SS, Meier CR, et al. Association between glycemic control and risk of fracture in diabetic patients: a nested case-control study. J Clin Endocrinol Metab. 2019; 104(5):1645–1654. PMID: 30657918.
Article6. Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. 2010; 33(7):1497–1499. PMID: 20413515.
Article7. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012; 31(5):652–658. PMID: 22414775.
Article8. Hida T, Ishiguro N, Shimokata H, Sakai Y, Matsui Y, Takemura M, et al. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int. 2013; 13(2):413–420. PMID: 22816427.
Article9. Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol. 2017; 176(3):R137–R157. PMID: 28049653.
Article10. Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995; 44(7):775–782. PMID: 7789645.
Article11. Samelson EJ, Demissie S, Cupples LA, Zhang X, Xu H, Liu CT, et al. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: Framingham HR-pQCT Study. J Bone Miner Res. 2018; 33(1):54–62. PMID: 28929525.
Article12. Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014; 25(7):1969–1973. PMID: 24718377.
Article13. Cipriani C, Colangelo L, Santori R, Renella M, Mastrantonio M, Minisola S, et al. The interplay between bone and glucose metabolism. Front Endocrinol (Lausanne). 2020; 11:122. PMID: 32265831.
Article14. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001; 44(2):129–146. PMID: 11270668.
Article15. Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014; 2:411–429. PMID: 24624331.
Article16. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414(6865):813–820. PMID: 11742414.
Article17. Kanazawa I, Sugimoto T. Diabetes mellitus-induced bone fragility. Intern Med. 2018; 57(19):2773–2785. PMID: 29780142.
Article18. Zayzafoon M, Stell C, Irwin R, McCabe LR. Extracellular glucose influences osteoblast differentiation and c-Jun expression. J Cell Biochem. 2000; 79(2):301–310. PMID: 10967557.
Article19. Bloemen V, de Vries TJ, Schoenmaker T, Everts V. Intercellular adhesion molecule-1 clusters during osteoclastogenesis. Biochem Biophys Res Commun. 2009; 385(4):640–645. PMID: 19501575.
Article20. Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997; 272(40):25190–25194. PMID: 9312132.
Article21. Okada Y, Morimoto I, Ura K, Watanabe K, Eto S, Kumegawa M, et al. Cell-to-Cell adhesion via intercellular adhesion molecule-1 and leukocyte function-associated antigen-1 pathway is involved in 1alpha,25(OH)2D3, PTH and IL-1alpha-induced osteoclast differentiation and bone resorption. Endocr J. 2002; 49(4):483–495. PMID: 12402981.
Article22. Kong L, Yang X. Study of intercellular adhesion molecule-1 (ICAM-1) in bone homeostasis. Curr Drug Targets. 2020; 21(4):328–337. PMID: 31560285.
Article23. Trackman PC. Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone. Matrix Biol. 2016; 52-54:7–18. PMID: 26772152.
Article24. Saito M, Marumo K. Effects of collagen crosslinking on bone material properties in health and disease. Calcif Tissue Int. 2015; 97(3):242–261. PMID: 25791570.
Article25. Weinberg E, Maymon T, Weinreb M. AGEs induce caspase-mediated apoptosis of rat BMSCs via TNFα production and oxidative stress. J Mol Endocrinol. 2014; 52(1):67–76. PMID: 24198288.
Article26. Suzuki R, Fujiwara Y, Saito M, Arakawa S, Shirakawa JI, Yamanaka M, et al. Intracellular accumulation of advanced glycation end products induces osteoblast apoptosis via endoplasmic reticulum stress. J Bone Miner Res. 2020; 35(10):1992–2003. PMID: 32427355.
Article27. Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007; 40(2):345–353. PMID: 17064973.
Article28. Okazaki K, Yamaguchi T, Tanaka K, Notsu M, Ogawa N, Yano S, et al. Advanced glycation end products (AGEs), but not high glucose, inhibit the osteoblastic differentiation of mouse stromal ST2 cells through the suppression of osterix expression, and inhibit cell growth and increasing cell apoptosis. Calcif Tissue Int. 2012; 91(4):286–296. PMID: 22903508.
Article29. Tanaka K, Yamagata K, Kubo S, Nakayamada S, Sakata K, Matsui T, et al. Glycolaldehyde-modified advanced glycation end-products inhibit differentiation of human monocytes into osteoclasts via upregulation of IL-10. Bone. 2019; 128:115034. PMID: 31421252.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Advanced Glycation End Products in Diabetic Vascular Complications
- Inhibition of advanced glycation end product formation by burdock root extract
- Letter: The Association between Serum Endogenous Secretory Receptor for Advanced Glycation End Products and Vertebral Fractures in Type 2 Diabetes (Endocrinol Metab 2012;27:289-94, Cheol Ho Lee et al.)
- Response: The Association between Serum Endogenous Secretory Receptor for Advanced Glycation End Products and Vertebral Fractures in Type 2 Diabetes (Endocrinol Metab 2012;27:289-94, Cheol Ho Lee et al.)
- Glyceraldehyde-Derived Advanced Glycation End Products Accumulate Faster Than N(ε)-(Carboxymethyl) Lysine