Korean J Gastroenterol.
2021 Sep;78(3):188-194. 10.4166/kjg.2021.045.
Epstein-Barr Virus-associated Mixed Lymphoepithelioma-like Carcinoma and Adenocarcinoma of the Gall Bladder: An Unusual Entity
- Affiliations
-
- 1Departments of Pathology, Govind Ballabh Pant Institute of Postgraduate Medical Education and Research, New Delhi, India
- 2Departments of Gastrointestinal Surgery, Govind Ballabh Pant Institute of Postgraduate Medical Education and Research, New Delhi, India
- KMID: 2520361
- DOI: http://doi.org/10.4166/kjg.2021.045
Abstract
- Schmincke described lymphoepithelioma as an undifferentiated carcinoma with abundant lymphoid stroma in the nasopharynx. Tumors with a similar histomorphology in extrapharyngeal areas have been referred to as lymphoepithelioma-like carcinoma (LELC). The association of an Ebstein-Barr virus (EBV) infection with lymphoepithelioma is well established in the nasopharynx but not so well at the extrapharyngeal sites. Only four cases of LELC have been reported in the gall bladder, of which all were negative for the EBV. This paper reports the first case of an EBV-associated mixed gall bladder carcinoma exhibiting a distinct phenotype of LELC and adenocarcinoma with mucinous differentiation. The EBV was confirmed by the strong granular membranous and cytoplasmic expression of LMP-1 (latent membrane protein-1) on immunohistochemistry and nuclear EBER RNA on chromogen in-situ hybridization in the tumor. This is the first case of LELC positive for EBV in the gall bladder. LELC has a more favorable prognosis than conventional adenocarcinoma or squamous cell carcinoma, irrespective of the site. Although a higher T stage and nodal metastasis were exceptional in the present case in contrast to the previous cases, the EBV-associated lymphocytic response might limit the disease spread and confer better overall survival and prognosis in these patients. Nevertheless, more prospective studies with a larger cohort will be needed to understand the pathogenesis, biological behavior, and prognosis of this rare entity.
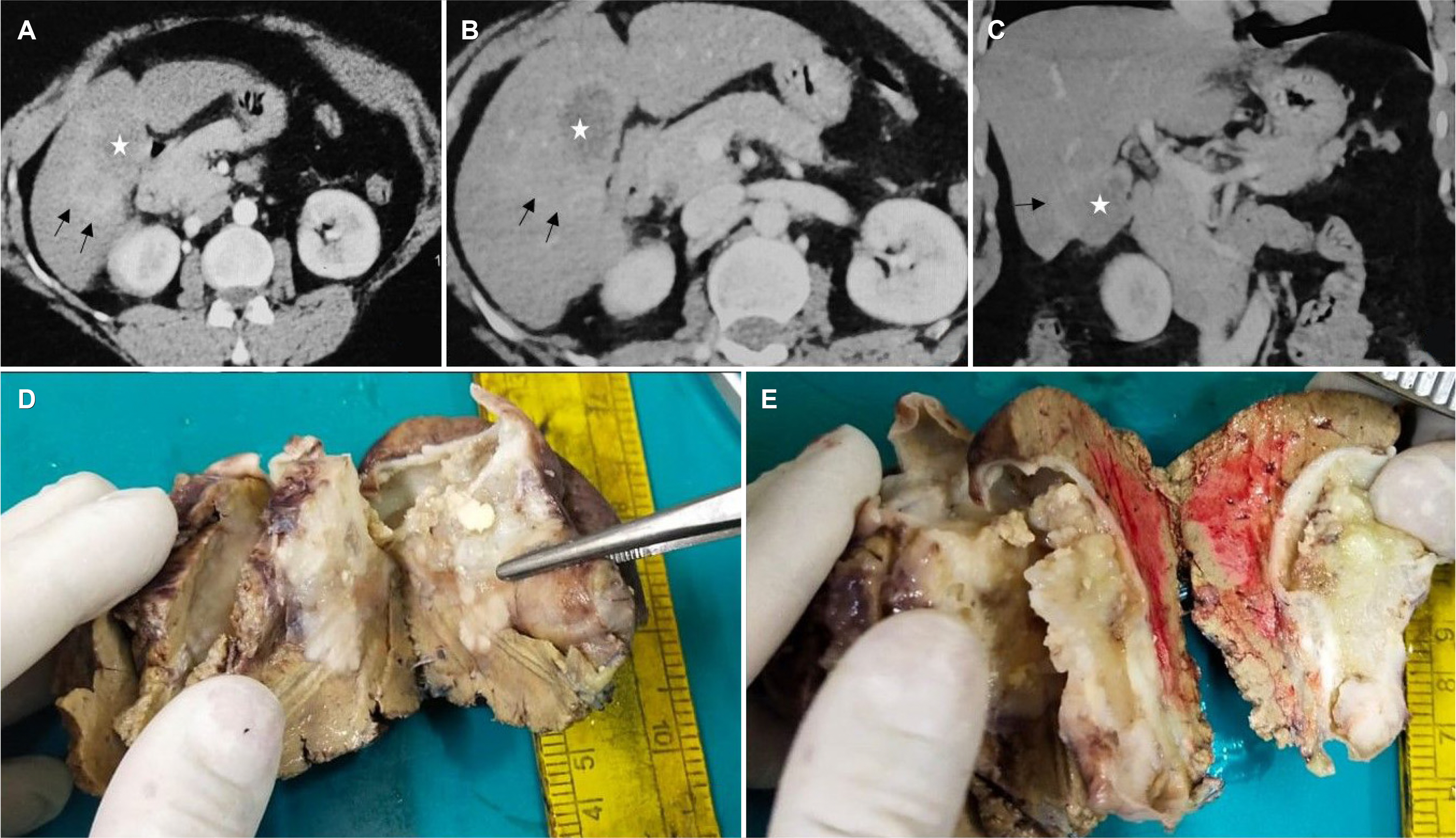
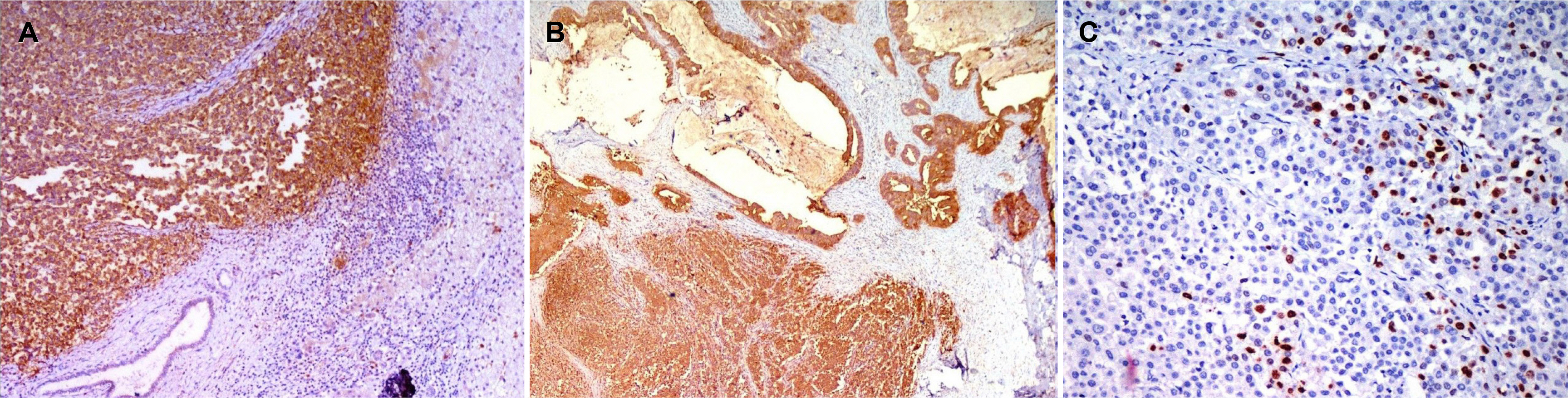
Figure
Reference
-
1. Schmincke A. 1921; Über lymphoepitheliale Geschwülste. Beitr pathol Anat Allg Pathol. 68:161–170.2. Choi NK, Lim SC. 2016; Mixed lymphoepithelioma-like carcinoma and adenocarcinoma of the gallbladder. Korean J Hepatobiliary Pancreat Surg. 20:148–151. DOI: 10.14701/kjhbps.2016.20.3.148. PMID: 27621754. PMCID: PMC5018954.
Article3. Todd DL, Ro JY, Gulley ML, Ayala AG. 1996; Lymphoepitheliomalike carcinoma of the gallbladder. Int J Surg Pathol. 4:183–187. DOI: 10.1177/106689699600400309.
Article4. Muto Y, Sho Y, Uchimura M. 1984; Carcinoma with lymphoid stroma of the gallbladder--a case report. Jpn J Surg. 14:399–404. DOI: 10.1007/BF02469548. PMID: 6513209.5. Sinha PK, Mangla V, Behari C, Rastogi A, Chattopdhyay TK. 2014; Lymphoepithelioma-like carcinoma: an unusual gall bladder tumour. Trop Gastroenterol. 35:182–183. DOI: 10.7869/tg.208. PMID: 26012324.
Article6. Carbone A, Micheau C. 1982; Pitfalls in microscopic diagnosis of undifferentiated carcinoma of nasopharyngeal type (lymphoepithelioma). Cancer. 50:1344–1351. DOI: 10.1002/1097-0142(19821001)50:7<1344::AID-CNCR2820500721>3.0.CO;2-O.7. Lauwers GY. Odze RD, Goldblum JR, editors. 2015. Epithelial Neoplasms of the Stomach. Odze and Goldblum surgical pathology of the GI tract, liver, biliary tract and pancreas. 3rd ed. Elsevier Health Sciences;Philadephia: p. 714.8. Herath CH, Chetty R. 2008; Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma. Arch Pathol Lab Med. 132:706–709. DOI: 10.5858/2008-132-706-EVLGC. PMID: 18384225.9. Zenger S, Gurbuz B, Can U, et al. 2019; Clinicopathological importance of colorectal medullary carcinoma. Eur Surg. 51:308–314. DOI: 10.1007/s10353-019-00613-3.
Article10. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. 2012. WHO classification of tumours of the breast. 4th ed. IARC Press;Lyon:11. Chen B, Chen X, Zhou P, et al. 2019; Primary pulmonary lymphoepithelioma-like carcinoma: a rare type of lung cancer with a favorable outcome in comparison to squamous carcinoma. Respir Res. 20:262. DOI: 10.1186/s12931-019-1236-2. PMID: 31752892. PMCID: PMC6873444.
Article12. Chou J, Lin YC, Kim J, et al. 2008; Nasopharyngeal carcinoma--review of the molecular mechanisms of tumorigenesis. Head Neck. 30:946–963. DOI: 10.1002/hed.20833. PMID: 18446839. PMCID: PMC3046044.
Article13. Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. 2009; Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 24:354–365. DOI: 10.1111/j.1440-1746.2009.05775.x. PMID: 19335785.
Article14. Cheng N, Hui DY, Liu Y, et al. 2015; Is gastric lymphoepithelioma-like carcinoma a special subtype of EBV-associated gastric carcinoma? New insight based on clinicopathological features and EBV genome polymorphisms. Gastric Cancer. 18:246–255. DOI: 10.1007/s10120-014-0376-9. PMID: 24771002.
Article15. Tsao SW, Tramoutanis G, Dawson CW, Lo AK, Huang DP. 2002; The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol. 12:473–487. DOI: 10.1016/S1044579X02000901. PMID: 12450733.
Article16. Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. 2003; Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 16:641–651. DOI: 10.1097/01.MP.0000076980.73826.C0. PMID: 12861059.
Article17. Thirunavukarasu P, Sathaiah M, Singla S, et al. 2010; Medullary carcinoma of the large intestine: a population based analysis. Int J Oncol. 37:901–907. DOI: 10.3892/ijo_00000741. PMID: 20811712. PMCID: PMC4127912.
Article18. Tamas EF, Nielsen ME, Schoenberg MP, Epstein JI. 2007; Lymphoepithelioma-like carcinoma of the urinary tract: a clinicopathological study of 30 pure and mixed cases. Mod Pathol. 20:828–834. DOI: 10.1038/modpathol.3800823. PMID: 17541442.
Article19. Qian XH, Zhou DK, Wang WL. 2020; Surgical treatment of Epstein-Barr virus-associated lymphoepithelioma-like carcinoma occurring in both the posterior mediastinum and liver: case report. Medicine (Baltimore). 99:e23610. DOI: 10.1097/MD.0000000000023610. PMID: 33350736. PMCID: PMC7769299.20. Song HJ, Srivastava A, Lee J, et al. 2010; Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 139:84–92.e2. DOI: 10.1053/j.gastro.2010.04.002. PMID: 20398662.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathology of Epstein-Barr Virus-Associated Gastric Carcinoma and Its Relationship to Prognosis
- Epstein-Barr Virus-Associated Lymphoepithelioma-Like Gastric Carcinoma Presenting as a Submucosal Mass: CT Findings with Pathologic Correlation
- A Case of Lymphoepithelioma-like Carcinoma Arising from the Parotid Gland
- A Case of Epstein-Barr Virus Infection with Gall Bladder and Common Bile Duct Stones in an Otherwise Healthy Child
- Mixed lymphoepithelioma-like carcinoma and adenocarcinoma of the gallbladder