Endocrinol Metab.
2021 Aug;36(4):823-834. 10.3803/EnM.2021.1074.
Non-Laboratory-Based Simple Screening Model for Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Developed Using Multi-Center Cohorts
- Affiliations
-
- 1Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Korea
- 2Department of Education and Training, Severance Hospital, Yonsei University College of Medicine, Korea
- 3Severance Health Check-up, Severance Hospital, Yonsei University Health System, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, Korea
- 6Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Division of Endocrinology and Metabolism, Department of Internal Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- KMID: 2519670
- DOI: http://doi.org/10.3803/EnM.2021.1074
Abstract
- Background
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent cause of chronic liver disease worldwide. Type 2 diabetes mellitus (T2DM) is a risk factor that accelerates NAFLD progression, leading to fibrosis and cirrhosis. Thus, here we aimed to develop a simple model to predict the presence of NAFLD based on clinical parameters of patients with T2DM.
Methods
A total of 698 patients with T2DM who visited five medical centers were included. NAFLD was evaluated using transient elastography. Univariate logistic regression analyses were performed to identify potential contributors to NAFLD, followed by multivariable logistic regression analyses to create the final prediction model for NAFLD.
Results
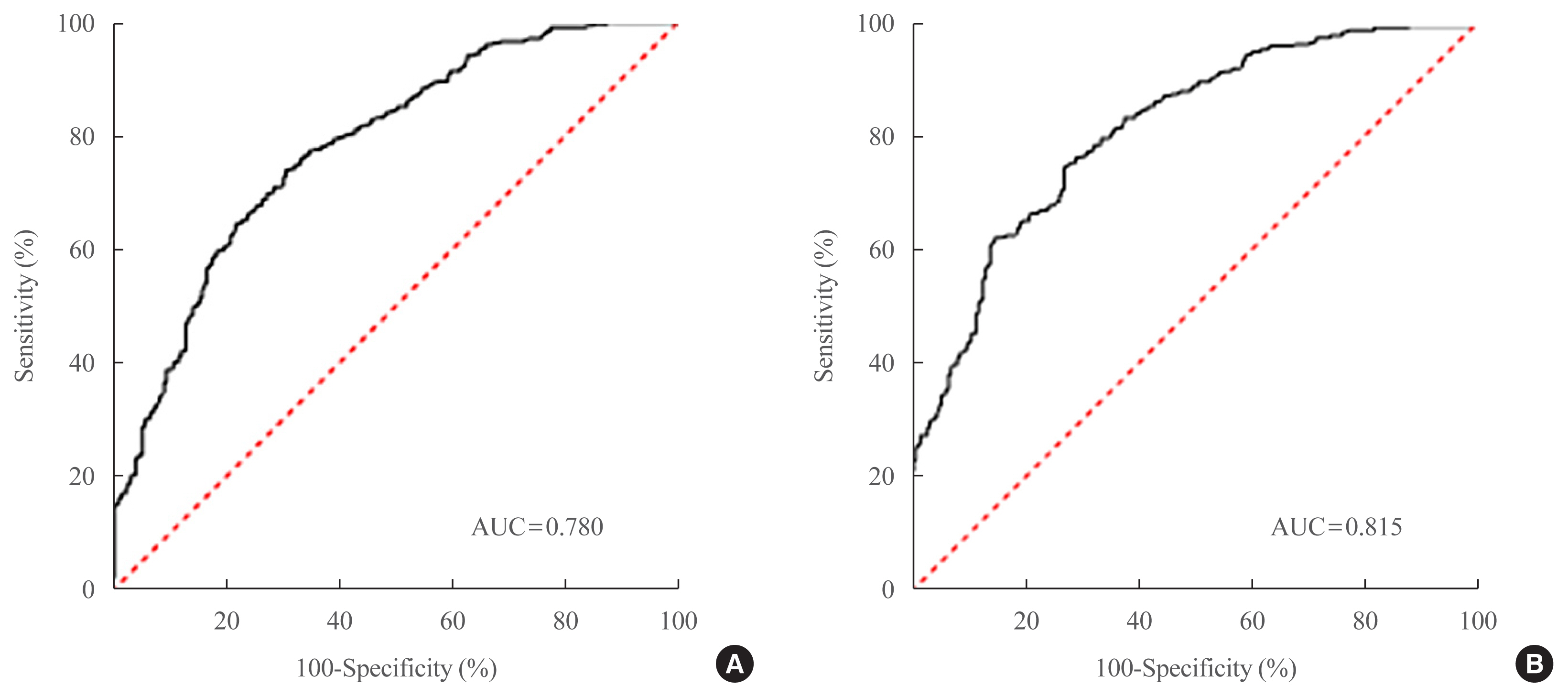
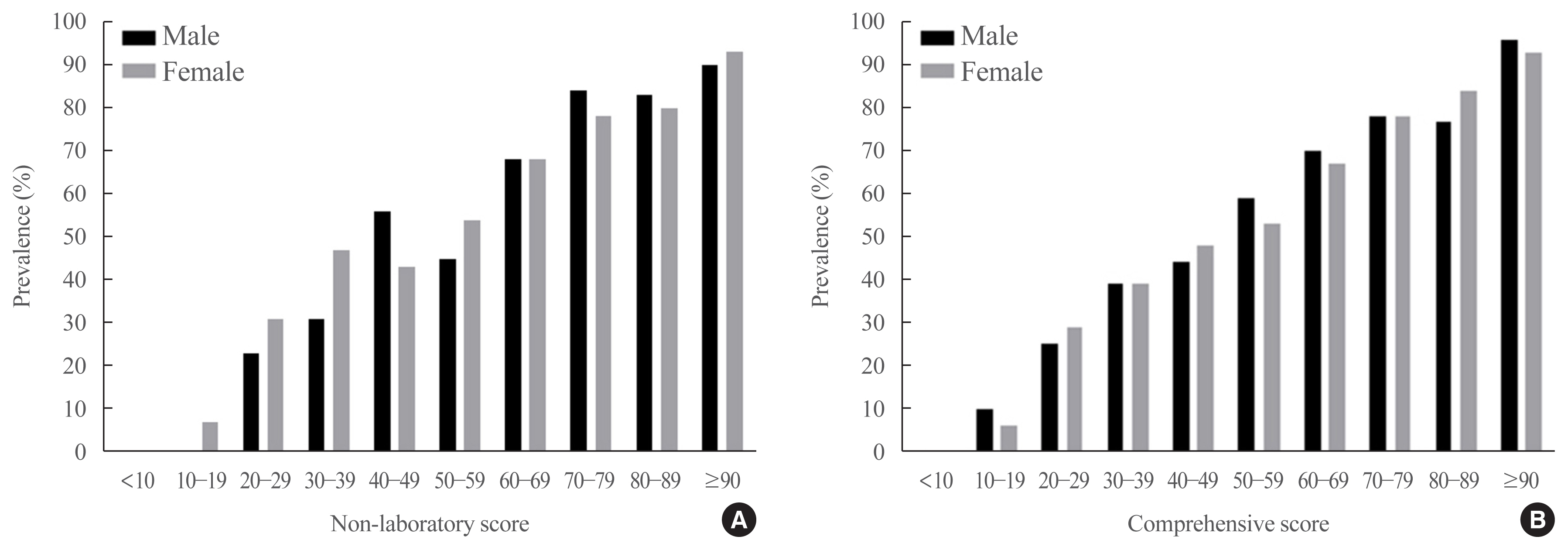
Two NAFLD prediction models were developed, with and without serum biomarker use. The non-laboratory model comprised six variables: age, sex, waist circumference, body mass index (BMI), dyslipidemia, and smoking status. For a cutoff value of ≥60, the prediction accuracy was 0.780 (95% confidence interval [CI], 0.743 to 0.817). The second comprehensive model showed an improved discrimination ability of up to 0.815 (95% CI, 0.782 to 0.847) and comprised seven variables: age, sex, waist circumference, BMI, glycated hemoglobin, triglyceride, and alanine aminotransferase to aspartate aminotransferase ratio. Our non-laboratory model showed non-inferiority in the prediction of NAFLD versus previously established models, including serum parameters.
Conclusion
The new models are simple and user-friendly screening methods that can identify individuals with T2DM who are at high-risk for NAFLD. Additional studies are warranted to validate these new models as useful predictive tools for NAFLD in clinical practice.
Keyword
Figure
Cited by 1 articles
-
Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
Inha Jung, Dae-Jeong Koo, Won-Young Lee
Diabetes Metab J. 2024;48(3):327-339. doi: 10.4093/dmj.2023.0350.
Reference
-
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84.
Article2. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018; 67:328–57.
Article3. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016; 64:1388–402.4. Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, et al. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab J. 2019; 43:31–45.
Article5. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019; 71:793–801.
Article6. Simeone JC, Bae JP, Hoogwerf BJ, Li Q, Haupt A, Ali AK, et al. Clinical course of nonalcoholic fatty liver disease: an assessment of severity, progression, and outcomes. Clin Epidemiol. 2017; 9:679–88.
Article7. Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008; 51:444–50.
Article8. Lee JI, Kim MC, Moon BS, Song YS, Han EN, Lee HS, et al. The relationship between 10-year cardiovascular risk calculated using the pooled cohort equation and the severity of non-alcoholic fatty liver disease. Endocrinol Metab (Seoul). 2016; 31:86–92.
Article9. Lee DH. Noninvasive evaluation of nonalcoholic fatty liver disease. Endocrinol Metab (Seoul). 2020; 35:243–59.
Article10. Lee YH, Bang H, Park YM, Bae JC, Lee BW, Kang ES, et al. Non-laboratory-based self-assessment screening score for non-alcoholic fatty liver disease: development, validation and comparison with other scores. PLoS One. 2014; 9:e107584.
Article11. Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, et al. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019; 43:398–406.
Article12. Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis. Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010; 36:1825–35.
Article13. Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007; 56:968–73.
Article14. de Ledinghen V, Vergniol J. Transient elastography (FibroScan). Gastroenterol Clin Biol. 2008; 32(6 Suppl 1):58–67.
Article15. Gaia S, Carenzi S, Barilli AL, Bugianesi E, Smedile A, Brunello F, et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011; 54:64–71.
Article16. Wong VW, Petta S, Hiriart JB, Camma C, Wong GL, Marra F, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017; 67:577–84.
Article17. Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008; 48:835–47.
Article18. Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993; 138:923–36.
Article19. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006; 6:33.
Article20. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010; 42:503–8.
Article21. Park YJ, Lim JH, Kwon ER, Kim HK, Jung MC, Seol KH, et al. Development and validation of a simple index system to predict nonalcoholic fatty liver disease. Korean J Hepatol. 2011; 17:19–26.
Article22. Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005; 42:44–52.
Article23. Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016; 20:205–14.
Article24. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006; 43(2 Suppl 1):S99–112.
Article25. Nam GE, Kim YH, Han K, Jung JH, Rhee EJ, Lee SS, et al. Obesity fact sheet in Korea, 2019: prevalence of obesity and abdominal obesity from 2009 to 2018 and social factors. J Obes Metab Syndr. 2020; 29:124–32.
Article26. Rhee EJ. Nonalcoholic fatty liver disease and diabetes: an epidemiological perspective. Endocrinol Metab (Seoul). 2019; 34:226–33.
Article27. Singh A, Dhaliwal AS, Singh S, Kumar A, Lopez R, Gupta M, et al. Awareness of nonalcoholic fatty liver disease is increasing but remains very low in a representative US cohort. Dig Dis Sci. 2020; 65:978–86.
Article28. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large U.S. claims database. Hepatology. 2018; 68:2230–8.
Article29. Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004; 2:262–5.
Article30. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017; 14:32–42.
Article31. Long MT, Pedley A, Colantonio LD, Massaro JM, Hoffmann U, Muntner P, et al. Development and validation of the framingham steatosis index to identify persons with hepatic steatosis. Clin Gastroenterol Hepatol. 2016; 14:1172–80.
Article32. Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009; 137:865–72.
Article33. Korea Centers for Disease Control and Prevention (KCDC). Korea National Health and Nutrition Examination Survey [Internet]. Cheongju: KCDC;2019. [cited 2021 Jul 27]. Available from: http://www.kdca.go.kr/ .34. Ayonrinde OT, Olynyk JK, Beilin LJ, Mori TA, Pennell CE, de Klerk N, et al. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology. 2011; 53:800–9.
Article35. Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006; 21(1 Pt 1):138–43.
Article36. Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007; 13:6419–24.
Article37. Shen L, Fan JG, Shao Y, Zeng MD, Wang JR, Luo GH, et al. Prevalence of nonalcoholic fatty liver among administrative officers in Shanghai: an epidemiological survey. World J Gastroenterol. 2003; 9:1106–10.
Article38. Zhang H, Liu Y, Wang L, Li Z, Zhang H, Wu J, et al. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013; 54:345–57.
Article39. Suzuki A, Abdelmalek MF. Nonalcoholic fatty liver disease in women. Womens Health (Lond). 2009; 5:191–203.
Article40. Park CY, Lim JY, Park HY. Age at natural menopause in Koreans: secular trends and influences thereon. Menopause. 2018; 25:423–9.
Article41. Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012; 56:234–40.
Article42. Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001; 38:263–355.
Article43. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015; 62:1148–55.
Article44. Yilmaz Y, Ergelen R, Akin H, Imeryuz N. Noninvasive detection of hepatic steatosis in patients without ultrasonographic evidence of fatty liver using the controlled attenuation parameter evaluated with transient elastography. Eur J Gastroenterol Hepatol. 2013; 25:1330–4.
Article45. Kunutsor SK. Gamma-glutamyltransferase: friend or foe within? Liver Int. 2016; 36:1723–34.46. Sanyal D, Mukherjee P, Raychaudhuri M, Ghosh S, Mukherjee S, Chowdhury S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J Endocrinol Metab. 2015; 19:597–601.
Article47. Zheng M, Chengliang C, Chen Y, Gopal N, Ramesh R, Jiao J. Diagnostic performance of fibroscan and computed tomography in 322 normal alanine aminotransferase non-obese non-alcoholic fatty liver disease patients diagnosed by ultrasound. Int J Clin Pract. 2020; 74:e13635.
Article48. Xian YX, Weng JP, Xu F. MAFLD vs. NAFLD: shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy. Chin Med J (Engl). 2020; 134:8–19.
Article49. Sesti G, Hribal ML, Fiorentino TV, Sciacqua A, Perticone F. Elevated 1 h postload plasma glucose levels identify adults with normal glucose tolerance but increased risk of non-alcoholic fatty liver disease. BMJ Open Diabetes Res Care. 2014; 2:e000016.50. Pu K, Wang Y, Bai S, Wei H, Zhou Y, Fan J, et al. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019; 19:51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent update on pathogenesis of nonalcoholic fatty liver disease
- Nonalcoholic Fatty Liver Disease in Children
- Nonalcoholic Fatty Liver Disease and Abdominal Fat Accumulation According to Vitamin D Status in Patients with Type 2 Diabetes
- Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments
- Comorbid diseases in nonalcoholic fatty liver disease