Ann Rehabil Med.
2021 Aug;45(4):274-283. 10.5535/arm.21078.
Mesenchymal Stem Cells Use in the Treatment of Tendon Disorders: A Systematic Review and Meta-Analysis of Prospective Clinical Studies
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea
- 2Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Orthopedic Surgery, Seoul National University College of Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea
- 4Department of Rehabilitation Medicine, Seoul National University College of Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea
- KMID: 2519634
- DOI: http://doi.org/10.5535/arm.21078
Abstract
Objective
To evaluate the efficacy and safety of mesenchymal stem cells (MSCs) therapy in patients with tendon disorders enrolled in prospective clinical studies.
Methods
We systematically searched prospective clinical studies that investigated the effects of MSC administration on human tendon disorders with at least a 6-month follow-up period in the PubMed-MEDLINE, EMBASE, and Cochrane Library databases. The primary outcome of interest was the change in pain on motion related to tendon disorders. Meta-regression analyses were performed to assess the relationship between MSC dose and pooled effect sizes in each cell dose.
Results
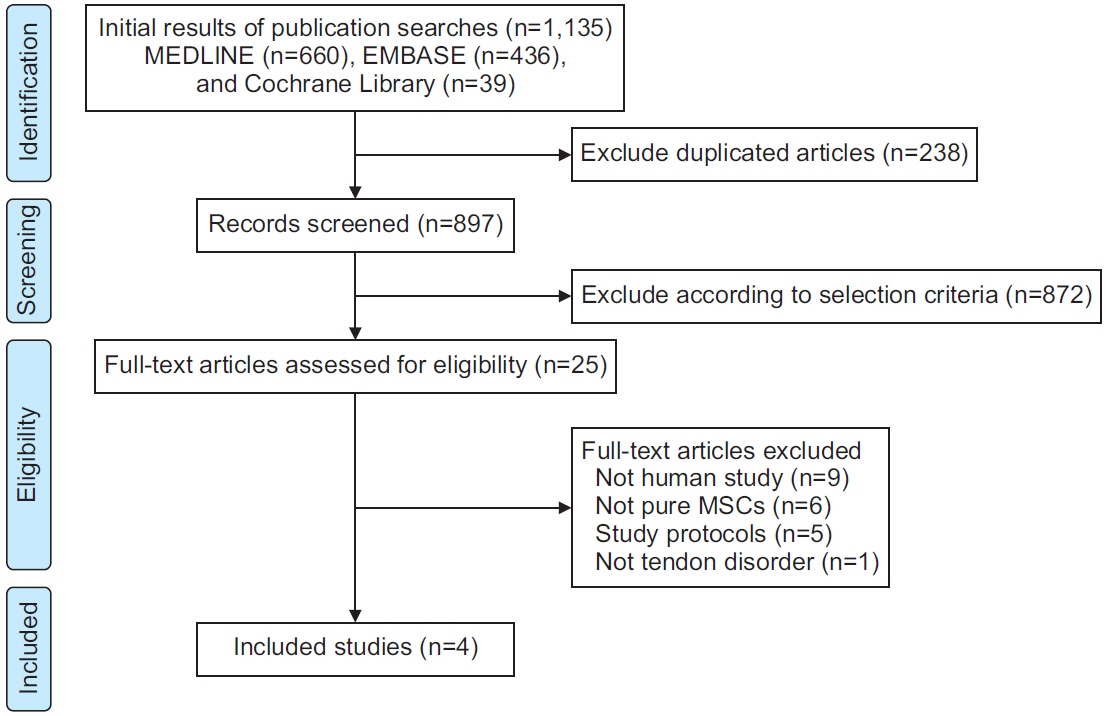
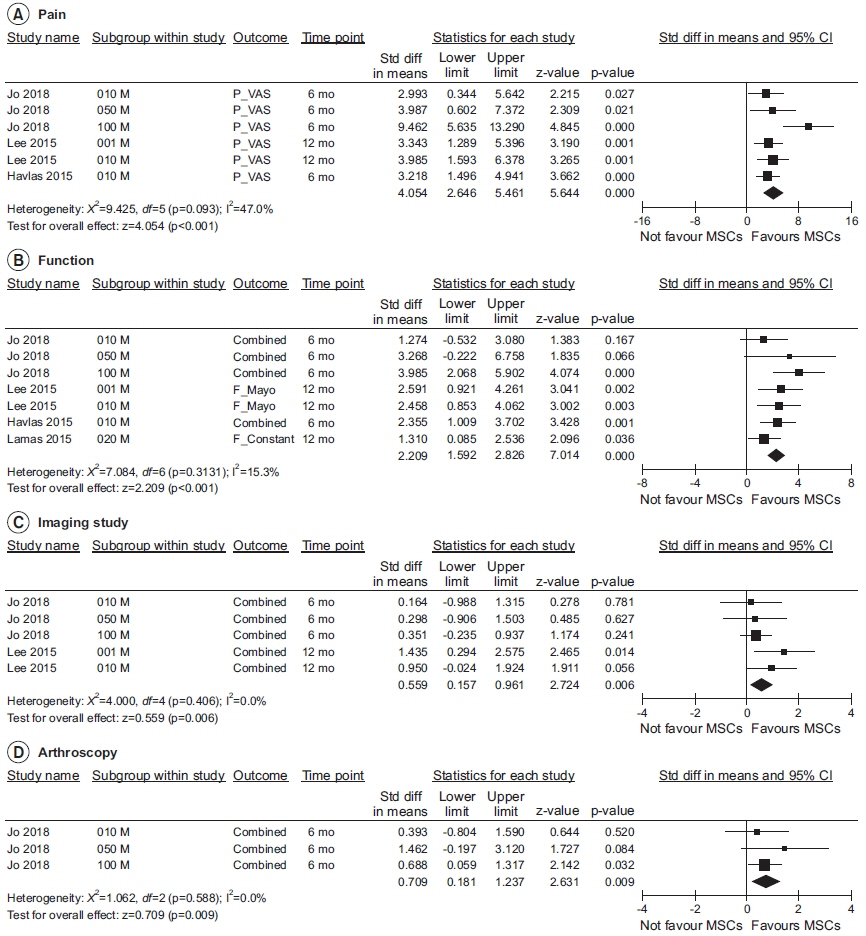
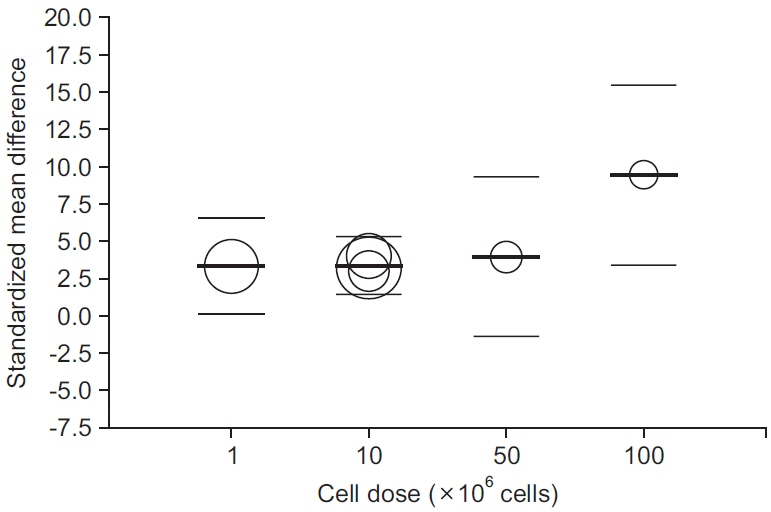
Four prospective clinical trials that investigated the effect of MSCs on tendon disorders were retrieved. MSCs showed a significant pooled effect size (overall Hedges’ g pooled standardized mean difference=1.868; 95% confidence interval, 1.274–2.462; p<0.001). The treatment with MSCs improved all the aspects analyzed, namely pain, functional scores, radiological parameters (magnetic resonance image or ultrasonography), and arthroscopic findings. In the meta-regression analysis, a significant cell dose-dependent response in pain relief (Q=9.06, p=0.029) was observed.
Conclusion
Our meta-analysis revealed that MSC therapy may improve pain, function, radiological, and arthroscopic parameters in patients with tendon disorders. A strong need for large-scale randomized controlled trials has emerged to confirm the long-term functional improvement and adverse effects of MSC therapies in tendon disorders.
Figure
Reference
-
1. Young M. Stem cell applications in tendon disorders: a clinical perspective. Stem Cells Int. 2012; 2012:637836.
Article2. Barboni B, Russo V, Gatta V, Bernabo N, Berardinelli P, Mauro A, et al. Therapeutic potential of hAECs for early Achilles tendon defect repair through regeneration. J Tissue Eng Regen Med. 2018; 12:e1594–e1608.
Article3. Liao GP, Choi Y, Vojnits K, Xue H, Aroom K, Meng F, et al. Tissue engineering to repair diaphragmatic defect in a rat model. Stem Cells Int. 2017; 2017:1764523.
Article4. Lee SY, Kwon B, Lee K, Son YH, Chung SG. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017; 45:1429–39.
Article5. Pas HI, Moen MH, Haisma HJ, Winters M. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017; 51:996–1002.
Article6. Pascual-Garrido C, Rolon A, Makino A. Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int. 2012; 2012:953510.
Article7. Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012; 20:373–7.
Article8. Lee SY, Kim W, Lim C, Chung SG. Treatment of lateral epicondylosis by using allogeneic adipose-derived mesenchymal stem cells: a pilot study. Stem Cells. 2015; 33:2995–3005.
Article9. Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014; 38:1811–8.
Article10. Havlas V, Kotaska J, Konicek P, Trc T, Konradova S, Koci Z, et al. [Use of cultured human autologous bone marrow stem cells in repair of a rotator cuff tear: preliminary results of a safety study]. Acta Chir Orthop Traumatol Cech. 2015; 82:229–34.11. Lamas JR, Garcia-Fernandez C, Tornero-Esteban P, Lopiz Y, Rodriguez-Rodriguez L, Ortega L, et al. Adverse effects of xenogenic scaffolding in the context of a randomized double-blind placebo-controlled study for repairing full-thickness rotator cuff tears. Trials. 2019; 20:387.
Article12. Wohn DY. Korea okays stem cell therapies despite limited peer-reviewed data. Nat Med. 2012; 18:329.
Article13. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015; 350:g7647.
Article14. Tsikopoulos K, Tsikopoulos I, Simeonidis E, Papathanasiou E, Haidich AB, Anastasopoulos N, et al. The clinical impact of platelet-rich plasma on tendinopathy compared to placebo or dry needling injections: a meta-analysis. Phys Ther Sport. 2016; 17:87–94.
Article15. Ranger TA, Wong AM, Cook JL, Gaida JE. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br J Sports Med. 2016; 50:982–9.
Article16. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–101.
Article17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.
Article18. Becker BJ. Synthesizing standardized mean‐change measures. Br J Math Stat Psychol. 1988; 41:257–78.
Article19. Jo CH, Chai JW, Jeong EC, Oh S, Kim PS, Yoon JY, et al. Intratendinous injection of autologous adipose tissuederived mesenchymal stem cells for the treatment of rotator cuff disease: a first-in-human trial. Stem Cells. 2018; 36:1441–50.
Article20. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008; 466:1539–54.
Article21. Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Arciero RA, Drissi H. Rapid isolation of human stem cells (connective tissue progenitor cells) from the proximal humerus during arthroscopic rotator cuff surgery. Am J Sports Med. 2010; 38:1438–47.
Article22. Nemoto M, Kizaki K, Yamamoto Y, Oonuma T, Hashizume K. Tenascin-C expression in equine tendonderived cells during proliferation and migration. J Equine Sci. 2013; 24:17–24.
Article23. Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004; 109:1543–9.
Article24. Yoon S, Kang JJ, Kim J, Park S, Kim JM. Efficacy and safety of intra-articular injections of hyaluronic acid combined with polydeoxyribonucleotide in the treatment of knee osteoarthritis. Ann Rehabil Med. 2019; 43:204–14.
Article25. Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014; 23:1045–59.
Article26. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016; 25:829–48.
Article27. Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012; 308:2369–79.28. Machova Urdzikova L, Sedlacek R, Suchy T, Amemori T, Ruzicka J, Lesny P, Havlas V, Sykova E, Jendelova P. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed Eng Online. 2014; 13:42.
Article29. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010; 376:1751–67.
Article30. Carballo CB, Lebaschi A, Rodeo SA. Cell-based approaches for augmentation of tendon repair. Tech Shoulder Elb Surg. 2017; 18:e6–e14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correction: Mesenchymal Stem Cells Use in the Treatment of Tendon Disorders: A Systematic Review and Meta-Analysis of Prospective Clinical Studies
- Systematic Review and Meta-analysis in Digestive Cancer Research
- Stem Cells for the Regeneration of Tendon and Ligament: A Perspective
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease
- Therapeutic Effects of Mesenchymal Stem Cells for Patients with Chronic Liver Diseases: Systematic Review and Meta-analysis