Int J Stem Cells.
2021 Aug;14(3):310-319. 10.15283/ijsc20184.
PSC-MSC-Derived Exosomes Protect against Kidney Fibrosis In Vivo and In Vitro through the SIRT6/β-Catenin Signaling Pathway
- Affiliations
-
- 1Department of Basic Medicine, School of Medicine, Xi’an Peihua University, Xi’an, China
- 2Department of Thoracic Surgery, Xi’an Chest Hospital, Xi’an, China
- KMID: 2519373
- DOI: http://doi.org/10.15283/ijsc20184
Abstract
- Background and Objectives
Chronic kidney disease (CKD) has a major impact on the quality of life of patients, and renal fibrosis is a critical pathological change in the disease. It is very important to control the process of renal fibrosis to improve the quality of life of patients with CKD. The pathological mechanism of renal fibrosis is very complicated, and the current treatment strategy also has many flaws.
Methods and Results
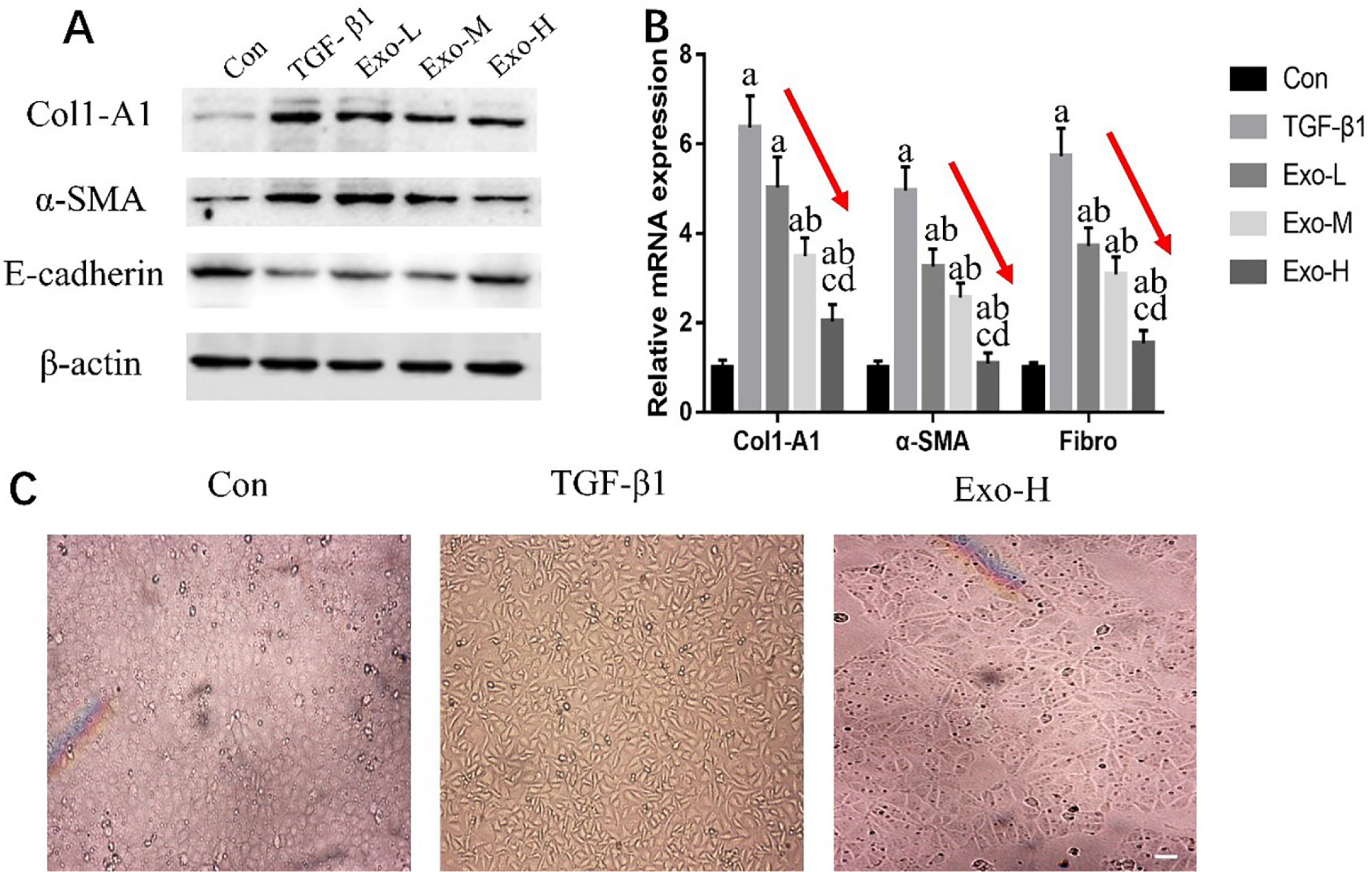
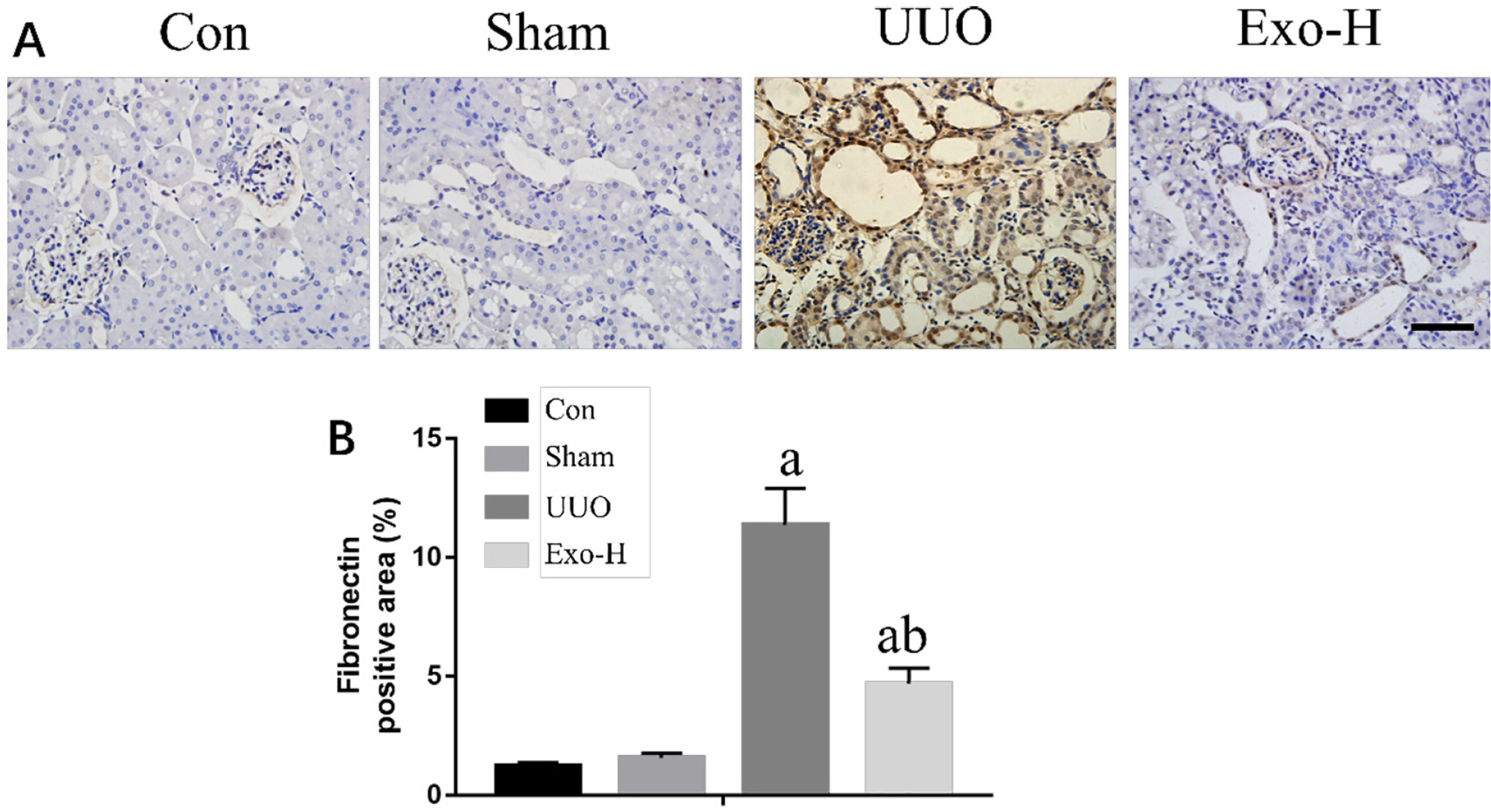
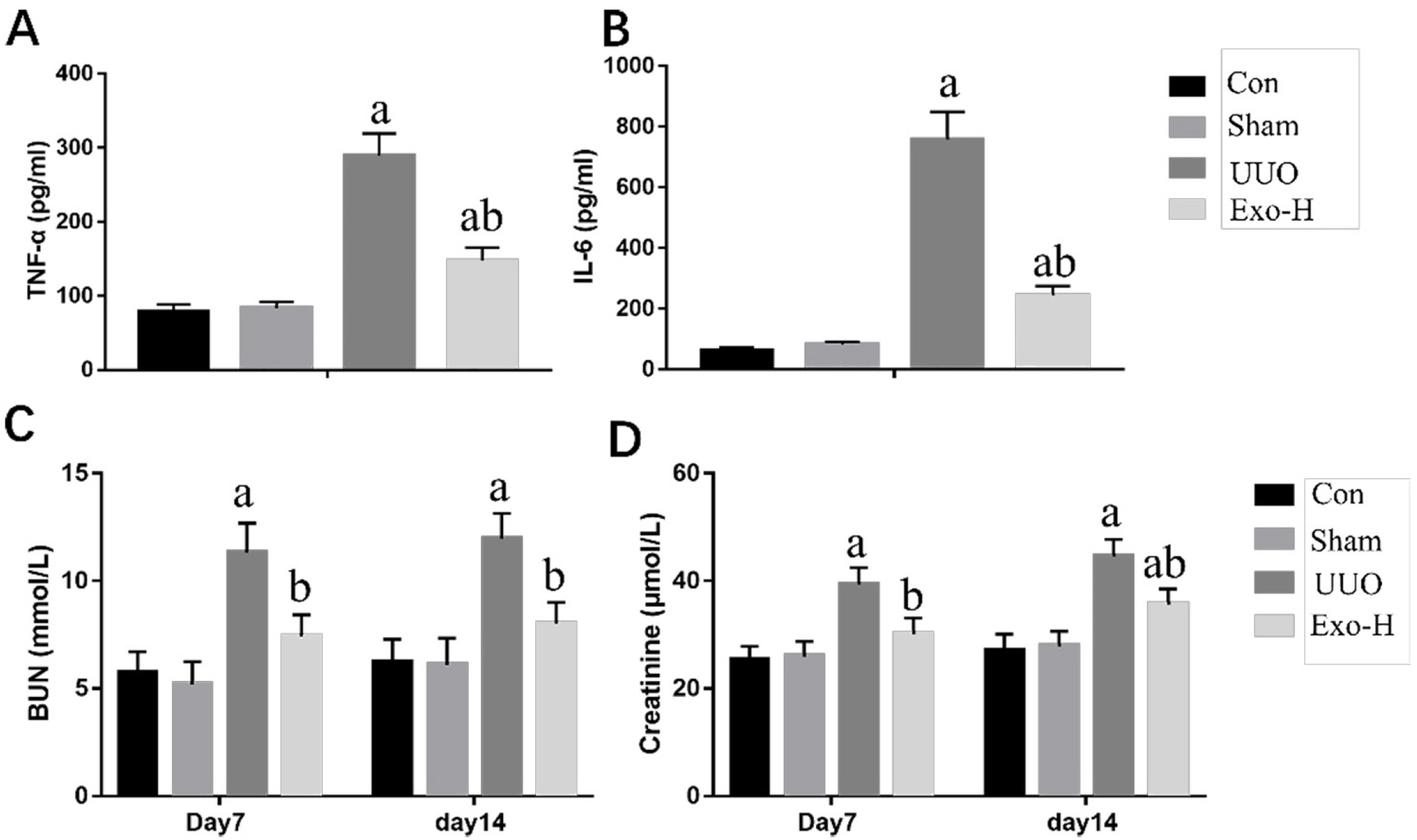
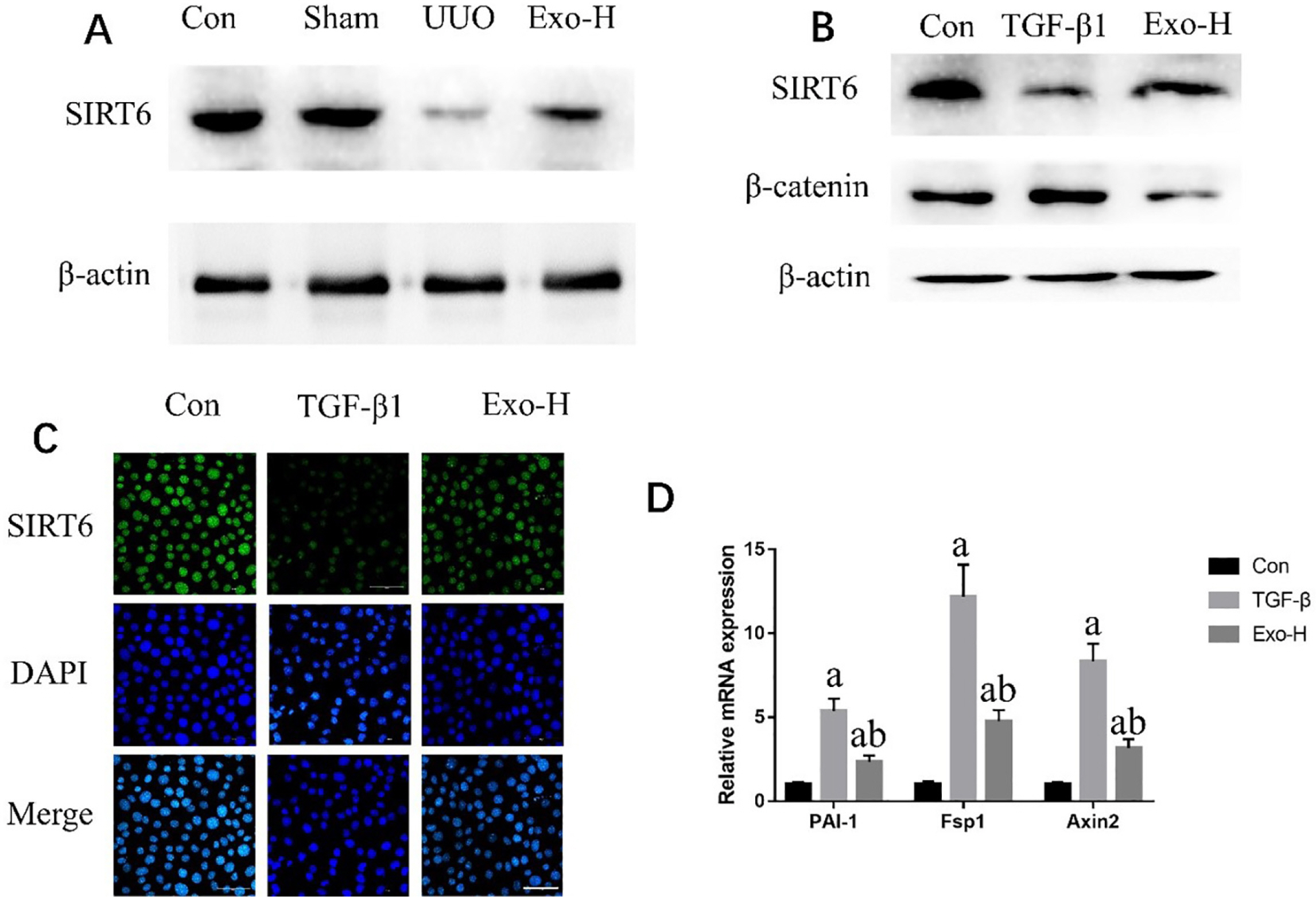
To explore a better treatment, we collected exosomes from pluripotent stem cell (PSC)-derived mesenchymal stem cells (MSC) and verified their therapeutic effect on renal fibrosis through In Vivo and In Vitro experiments. In this study, we found that PSC-MSC-derived comes could prevent the epithelial differentiation of NRK-52E cells, and with increasing exosome concentrations, the effect was improved. Furthermore, PSC-MSC-derived exosomes could reduce the pathological process of renal fibrosis, reduce inflammatory reactions and improve renal function in UUO mice. Moreover, the protective effect of exosomes against renal fibrosis may be achieved by increasing the expression of SIRT6 and decreasing the expression of β-catenin and its downstream products.
Conclusions
These findings suggest the possibility of PSC-MSC-derived exosomes as a new, effective therapeutic tool for kidney fibrosis.
Keyword
Figure
Reference
-
References
1. Wang Y, Xing QQ, Tu JK, Tang WB, Yuan XN, Xie YY, Wang W, Peng ZZ, Huang L, Xu H, Qin J, Xiao XC, Tao LJ, Yuan QJ. 2019; Involvement of hydrogen sulfide in the progression of renal fibrosis. Chin Med J (Engl). 132:2872–2880. DOI: 10.1097/CM9.0000000000000537. PMID: 31856060. PMCID: PMC6940064.
Article2. Bai M, Lei J, Wang S, Ding D, Yu X, Guo Y, Chen S, Du Y, Li D, Zhang Y, Huang S, Jia Z, Zhang A. 2019; BMP1 inhibitor UK383,367 attenuates renal fibrosis and inflammation in CKD. Am J Physiol Renal Physiol. 317:F1430–F1438. DOI: 10.1152/ajprenal.00230.2019. PMID: 31545926.
Article3. Owsiany MT, Hawley CE, Triantafylidis LK, Paik JM. 2019; Opioid management in older adults with chronic kidney disease: a review. Am J Med. 132:1386–1393. DOI: 10.1016/j.amjmed.2019.06.014. PMID: 31295441. PMCID: PMC6917891.
Article4. Gwon MG, An HJ, Kim JY, Kim WH, Gu H, Kim HJ, Leem J, Jung HJ, Park KK. 2020; Anti-fibrotic effects of synthetic TGF-β1 and Smad oligodeoxynucleotide on kidney fibrosis in vivo and in vitro through inhibition of both epithelial dedifferentiation and endothelial-mesenchymal tran-sitions. FASEB J. 34:333–349. DOI: 10.1096/fj.201901307RR. PMID: 31914629.
Article5. Chen YT, Hsu H, Lin CC, Pan SY, Liu SY, Wu CF, Tsai PZ, Liao CT, Cheng HT, Chiang WC, Chen YM, Chu TS, Lin SL. 2020; Inflammatory macrophages switch to CCL17-expressing phenotype and promote peritoneal fibrosis. J Pathol. 250:55–66. DOI: 10.1002/path.5350. PMID: 31579932.
Article6. Chow BSM, Kocan M, Shen M, Wang Y, Han L, Chew JY, Wang C, Bosnyak S, Mirabito-Colafella KM, Barsha G, Wigg B, Johnstone EKM, Hossain MA, Pfleger KDG, Denton KM, Widdop RE, Summers RJ, Bathgate RAD, Hewitson TD, Samuel CS. 2019; AT1R-AT2R-RXFP1 functional crosstalk in myofibroblasts: impact on the therapeutic targeting of renal and cardiac fibrosis. J Am Soc Nephrol. 30:2191–2207. DOI: 10.1681/ASN.2019060597. PMID: 31511361. PMCID: PMC6830801.
Article7. Zhao X, Kwan JYY, Yip K, Liu PP, Liu FF. 2020; Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 19:57–75. DOI: 10.1038/s41573-019-0040-5. PMID: 31548636.
Article8. Uccelli A, Moretta L, Pistoia V. 2008; Mesenchymal stem cells in health and disease. Nat Rev Immunol. 8:726–736. DOI: 10.1038/nri2395. PMID: 19172693.
Article9. Zhang S, Hu B, Liu W, Wang P, Lv X, Chen S, Liu H, Shao Z. 2020; Articular cartilage regeneration: the role of endogenous mesenchymal stem/progenitor cell recruitment and migration. Semin Arthritis Rheum. 50:198–208. DOI: 10.1016/j.semarthrit.2019.11.001. PMID: 31767195.
Article10. Harrell CR, Markovic BS, Fellabaum C, Arsenijevic N, Djonov V, Volarevic V. 2020; The role of Interleukin 1 receptor antagonist in mesenchymal stem cell-based tissue repair and regeneration. Biofactors. 46:263–275. DOI: 10.1002/biof.1587. PMID: 31755595.
Article11. Hoogduijn MJ, Lombardo E. 2019; Mesenchymal stromal cells anno 2019: dawn of the therapeutic era? Concise review. Stem Cells Transl Med. 8:1126–1134. DOI: 10.1002/sctm.19-0073. PMID: 31282113. PMCID: PMC6811696.
Article12. Zhao L, Hu C, Zhang P, Jiang H, Chen J. 2020; Melatonin preconditioning is an effective strategy for mesenchymal stem cell-based therapy for kidney disease. J Cell Mol Med. 24:25–33. DOI: 10.1111/jcmm.14769. PMID: 31747719. PMCID: PMC6933322.
Article13. Cao J, Wang B, Tang T, Lv L, Ding Z, Li Z, Hu R, Wei Q, Shen A, Fu Y, Liu B. 2020; Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 11:206. DOI: 10.1186/s13287-020-01719-2. PMID: 32460853. PMCID: PMC7251891.
Article14. Peng KY, Lee YW, Hsu PJ, Wang HH, Wang Y, Liou JY, Hsu SH, Wu KK, Yen BL. 2016; Human pluripotent stem cell (PSC)-derived mesenchymal stem cells (MSCs) show potent neurogenic capacity which is enhanced with cytoskeletal rearrangement. Oncotarget. 7:43949–43959. DOI: 10.18632/oncotarget.9947. PMID: 27304057. PMCID: PMC5190070.
Article15. Zhang J, Liu X, Li H, Chen C, Hu B, Niu X, Li Q, Zhao B, Xie Z, Wang Y. 2016; Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 7:136. DOI: 10.1186/s13287-016-0391-3. PMID: 27650895. PMCID: PMC5028974.
Article16. Sfougataki I, Varela I, Stefanaki K, Karagiannidou A, Roubelakis MG, Kalodimou V, Papathanasiou I, Traeger-Synodinos J, Kitsiou-Tzeli S, Kanavakis E, Kitra V, Tsezou A, Tzetis M, Goussetis E. 2020; Proliferative and chondrogenic potential of mesenchymal stromal cells from pluripotent and bone marrow cells. Histol Histopathol. 35:1415–1426. DOI: 10.14670/HH-18-259. PMID: 32959885.17. Muraoka H, Hasegawa K, Sakamaki Y, Minakuchi H, Kawaguchi T, Yasuda I, Kanda T, Tokuyama H, Wakino S, Itoh H. 2019; Role of Nampt-Sirt6 axis in renal proximal tubules in extracellular matrix deposition in diabetic nephro-pathy. Cell Rep. 27:199–212.e5. DOI: 10.1016/j.celrep.2019.03.024. PMID: 30943401.
Article18. Liu M, Liang K, Zhen J, Zhou M, Wang X, Wang Z, Wei X, Zhang Y, Sun Y, Zhou Z, Su H, Zhang C, Li N, Gao C, Peng J, Yi F. 2017; Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun. 8:413. DOI: 10.1038/s41467-017-00498-4. PMID: 28871079. PMCID: PMC5583183.
Article19. Cai J, Liu Z, Huang X, Shu S, Hu X, Zheng M, Tang C, Liu Y, Chen G, Sun L, Liu H, Liu F, Cheng J, Dong Z. 2020; The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking β-catenin target gene expression. Kidney Int. 97:106–118. DOI: 10.1016/j.kint.2019.08.028. PMID: 31787254.
Article20. Kalluri R, LeBleu VS. 2020; The biology, function, and biomedical applications of exosomes. Science. 367:eaau6977. DOI: 10.1126/science.aau6977. PMID: 32029601. PMCID: PMC7717626.
Article21. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Cordeiro-da-Silva A. 2018; Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 7:1535750. DOI: 10.1080/20013078.2018.1535750. PMID: 30637094. PMCID: PMC6322352.
Article22. Sun N, Zhai L, Li H, Shi LH, Yao Z, Zhang B. 2016; Angiotensin-converting enzyme inhibitor (ACEI)-mediated amelioration in renal fibrosis involves suppression of mast cell degranulation. Kidney Blood Press Res. 41:108–118. DOI: 10.1159/000368549. PMID: 26881856.
Article23. Tan SHS, Wong JRY, Sim SJY, Tjio CKE, Wong KL, Chew JRJ, Hui JHP, Toh WS. 2020; Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater Today Bio. 7:100067. DOI: 10.1016/j.mtbio.2020.100067. PMID: 32695985. PMCID: PMC7364174.
Article24. Chen J, Ren S, Duscher D, Kang Y, Liu Y, Wang C, Yuan M, Guo G, Xiong H, Zhan P, Wang Y, Machens HG, Chen Z. 2019; Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J Cell Physiol. 234:23097–23110. DOI: 10.1002/jcp.28873. PMID: 31124125.
Article25. Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. 2019; LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 10:393. DOI: 10.1186/s13287-019-1522-4. PMID: 31847890. PMCID: PMC6918658.
Article26. Thongboonkerd V. 2020; Roles for exosome in various kidney diseases and disorders. Front Pharmacol. 10:1655. DOI: 10.3389/fphar.2019.01655. PMID: 32082158. PMCID: PMC7005210.
Article27. Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. 2013; Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 4:34. DOI: 10.1186/scrt194. PMID: 23618405. PMCID: PMC3707035.
Article28. Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, Fujimiya M. 2016; Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep. 6:34842. DOI: 10.1038/srep34842. PMID: 27721418. PMCID: PMC5056395.
Article29. Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, Brennan EP, Wilkinson-Berka JL, Wise AF, Ricardo SD. 2016; Mesenchymal stem cells deliver exogenous microrna-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 24:1290–1301. DOI: 10.1038/mt.2016.90. PMID: 27203438. PMCID: PMC5088767.
Article30. Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, Kourembanas S. 2019; Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 4:e128060. DOI: 10.1172/jci.insight.128060. PMID: 31581150. PMCID: PMC6948760.
Article31. Hao L, Bang IH, Wang J, Mao Y, Yang JD, Na SY, Seo JK, Choi HS, Bae EJ, Park BH. 2020; ERRγ suppression by Sirt6 alleviates cholestatic liver injury and fibrosis. JCI Insight. 5:e137566. DOI: 10.1172/jci.insight.137566. PMID: 32701506. PMCID: PMC7526444.
Article32. Rao P, Pang M, Qiao X, Yu H, Wang H, Yang Y, Ren X, Hu M, Chen T, Cao Q, Wang Y, Khushi M, Zhang G, Wang YM, Heok P'ng C, Nankivell B, Lee VW, Alexander SI, Zheng G, Harris DC. 2019; Promotion of β-catenin/Foxo1 signaling ameliorates renal interstitial fibrosis. Lab Invest. 99:1689–1701. DOI: 10.1038/s41374-019-0276-z. PMID: 31243340.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The crosstalk of Wnt/β-catenin signaling and p53 in acute kidney injury and chronic kidney disease
- Mesenchymal Stem Cell-Derived Exosomes are Effective for Radiation Enteritis and Essential for the Proliferation and Differentiation of Lgr5+ Intestinal Epithelial Stem Cells by Regulating Mir-195/Akt/b-Catenin Pathway
- Bone marrow mesenchymal stem cell exosomes suppress JAK/STAT signaling pathway in acute myeloid leukemia in vitro
- Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases
- Mest Attenuates CCl4-Induced Liver Fibrosis in Rats by Inhibiting the Wnt/beta-Catenin Signaling Pathway