J Korean Med Sci.
2021 Aug;36(33):e212. 10.3346/jkms.2021.36.e212.
Patient-Reported Outcomes Measurement Information System: Translation and Linguistic Validation of Six Profile Domains for Korean Adults
- Affiliations
-
- 1Center for Clinical Epidemiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Clinical Research Design and Evaluation, SAIHST, Sungkyunkwan University, Seoul, Korea
- 3Cancer Education Center, Samsung Medical Center, Seoul, Korea
- 4Department of Digital Health, SAIHST, Sungkyunkwan University, Seoul, Korea
- 5Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Orthopedic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 8Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 9Division of Hematology/Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2519352
- DOI: http://doi.org/10.3346/jkms.2021.36.e212
Abstract
- Background
The purpose of the study was to translate and linguistically validate a Korean language version of the PROMIS (K-PROMIS) for the six profile adult domains: Fatigue, Pain Intensity, Pain Interference, Physical Function, Sleep Disturbance, and Ability to Participate in Social Roles and Activities.
Methods
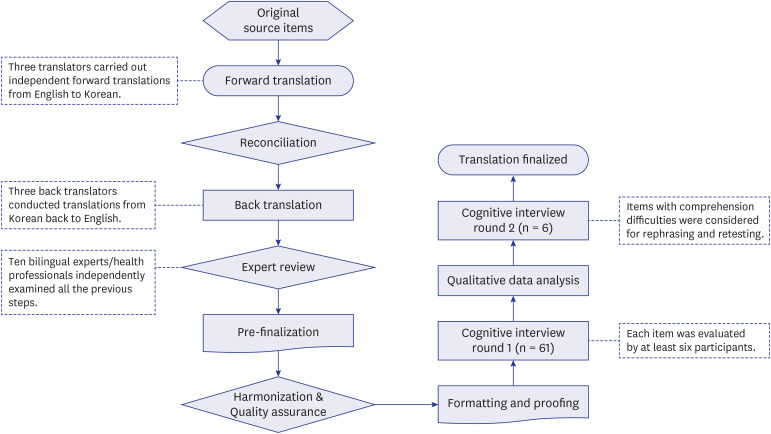
A total of 268 items were translated into Korean according to the Functional Assessment of Chronic Illness Therapy multilingual translation methodology. Participants first completed approximately 27 to 35 items and were then interviewed to evaluate the conceptual equivalence of the translation to the original English language source. The K-PROMIS items that met the a priori threshold of ≥ 20% of respondents with comprehension difficulties in the cognitive interview.
Results
54 of the 268 items were identified as difficult items to comprehend for at least 20% of respondents in Round 1. The most frequently identified K-PROMIS domain on difficult items to comprehend was the Physical function (24.5%). Most items with linguistic difficulties were Fatigue and Physical function. Cultural difficulties were only included the Physical function and Ability to Participate in Social Roles and Activities domains. 25 of 54 items were slightly revised, and then these revised items were tested with additional six participants in Round 2, and most participants had no problems to understand modified items.
Conclusion
The six profile adult domains of K-PROMIS have been linguistically validated. Further psychometric validation of the K-PROMIS items will provide additional information of meaningful outcomes for chronic disease and clinical setting.
Keyword
Figure
Reference
-
1. U.S. Food and Drug Administration. Guidance for industry: patient-reported outcomes measures: use in medical product development to support labeling claims. Updated 2009. Accessed December 23, 2019. https://www.fda.gov/media/77832/download.2. Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007; 25(32):5121–5127. PMID: 17991931.
Article3. Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013; 13(1):211. PMID: 23758898.
Article4. Howell D, Molloy S, Wilkinson K, Green E, Orchard K, Wang K, et al. Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol. 2015; 26(9):1846–1858. PMID: 25888610.
Article5. Denis F, Basch E, Septans AL, Bennouna J, Urban T, Dueck AC, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA. 2019; 321(3):306–307. PMID: 30667494.
Article6. Ishaque S, Karnon J, Chen G, Nair R, Salter AB. A systematic review of randomised controlled trials evaluating the use of patient-reported outcome measures (PROMs). Qual Life Res. 2019; 28(3):567–592. PMID: 30284183.
Article7. Snyder CF, Jensen RE, Segal JB, Wu AW. Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research. Med Care. 2013; 51(8):Suppl 3. S73–S79. PMID: 23774513.8. Ahmed S, Berzon RA, Revicki DA, Lenderking WR, Moinpour CM, Basch E, et al. The use of patient-reported outcomes (PRO) within comparative effectiveness research: implications for clinical practice and health care policy. Med Care. 2012; 50(12):1060–1070. PMID: 22922434.9. Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res. 2012; 21(8):1305–1314. PMID: 22048932.
Article10. Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-reported outcomes measurement information system (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007; 45(5):Suppl 1. S3–11.11. Riley WT, Rothrock N, Bruce B, Christodolou C, Cook K, Hahn EA, et al. Patient-reported outcomes measurement information system (PROMIS) domain names and definitions revisions: further evaluation of content validity in IRT-derived item banks. Qual Life Res. 2010; 19(9):1311–1321. PMID: 20593306.
Article12. Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010; 63(11):1179–1194. PMID: 20685078.
Article13. Haan EA, Terwee CB, Van Wier MF, Willigenburg NW, Van Deurzen DF, Pisters MF, et al. Translation, cross-cultural and construct validity of the Dutch-Flemish PROMIS® upper extremity item bank v2.0. Qual Life Res. 2020; 29(4):1123–1135. PMID: 31894506.
Article14. Choi H, Kim C, Ko H, Park CG. Translation and validation of the Korean version of PROMIS® pediatric and parent proxy measures for emotional distress. J Patient Rep Outcomes. 2019; 3(1):36. PMID: 31222609.
Article15. Devine J, Schröder LA, Metzner F, Klasen F, Moon J, Herdman M, et al. Correction to: Translation and cross-cultural adaptation of eight pediatric PROMIS® item banks into Spanish and German. Qual Life Res. 2018; 27(11):3057. PMID: 30145627.
Article16. Westmoreland K, Reeve BB, Amuquandoh A, van der Gronde T, Manthalu O, Correia H, et al. Translation, psychometric validation, and baseline results of the Patient-Reported Outcomes Measurement Information System (PROMIS) pediatric measures to assess health-related quality of life of patients with pediatric lymphoma in Malawi. Pediatr Blood Cancer. 2018; 65(11):e27353. PMID: 30015407.
Article17. Devine J, Schröder LA, Metzner F, Klasen F, Moon J, Herdman M, et al. Translation and cross-cultural adaptation of eight pediatric PROMIS® item banks into Spanish and German. Qual Life Res. 2018; 27(9):2415–2430. PMID: 29766439.
Article18. Schnohr CW, Rasmussen CL, Langberg H, Bjørner JB. Danish translation of a physical function item bank from the Patient-Reported Outcome Measurement Information System (PROMIS). Pilot Feasibility Stud. 2017; 3(1):29. PMID: 28573045.
Article19. Jakob T, Nagl M, Gramm L, Heyduck K, Farin E, Glattacker M. Psychometric properties of a German translation of the PROMIS® depression item bank. Eval Health Prof. 2017; 40(1):106–120. PMID: 26272632.
Article20. Haverman L, Grootenhuis MA, Raat H, van Rossum MA, van Dulmen-den Broeder E, Hoppenbrouwers K, et al. Dutch-Flemish translation of nine pediatric item banks from the Patient-Reported Outcomes Measurement Information System (PROMIS)®. Qual Life Res. 2016; 25(3):761–765. PMID: 25820548.
Article21. Silva EC, Pinto Rde M, Mendonca TM, Silva CH. Brazilian-Portuguese translation and cultural adaptation of the sleep and wake disturbances domains of the Patient-reported-outcomes measurement information system (PROMIS). Cad Saude Publica. 2014; 30(7):1391–1401. PMID: 25166937.22. Alves FS, Pinto RM, Mendonça TM, Silva CH. Portuguese-language translation and cross-cultural adaptation of the fatigue domain of patient-reported-outcomes measurement information system (PROMIS). Cad Saude Publica. 2014; 30(5):1103–1110. PMID: 24936825.23. Terwee CB, Roorda LD, de Vet HC, Dekker J, Westhovens R, van Leeuwen J, et al. Dutch-Flemish translation of 17 item banks from the patient-reported outcomes measurement information system (PROMIS). Qual Life Res. 2014; 23(6):1733–1741. PMID: 24402179.
Article24. Bevans M, Ross A, Cella D. Patient-reported outcomes measurement information system (PROMIS): efficient, standardized tools to measure self-reported health and quality of life. Nurs Outlook. 2014; 62(5):339–345. PMID: 25015409.
Article25. Fischer HF, Wahl I, Nolte S, Liegl G, Brähler E, Löwe B, et al. Language-related differential item functioning between English and German PROMIS depression items is negligible. Int J Methods Psychiatr Res. 2017; 26(4):e1530.
Article26. Choi JS, Park YS, Kim JA, Park CS. International trends on patient-reported outcome measures for improving care quality and its implication for South Korea: focus on OECD PaRIS. Qual Improv Health Care. 2019; 25(1):11–28.
Article27. Bonomi AE, Cella DF, Hahn EA, Bjordal K, Sperner-Unterweger B, Gangeri L, et al. Multilingual translation of the functional assessment of cancer therapy (FACT) quality of life measurement system. Qual Life Res. 1996; 5(3):309–320. PMID: 8763799.
Article28. Eremenco SL, Cella D, Arnold BJ. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Eval Health Prof. 2005; 28(2):212–232. PMID: 15851774.
Article29. Willis GB. Cognitive Interviewing: a Tool for Improving Questionnaire Design. New York, NY, USA: SAGE Publications;2004.30. Solorio R, Ayala NC, Paez E, Skalicky AM, Morales LS. Use of cognitive interviews to adapt PROMIS measurement items for Spanish speakers living with HIV. Aids Res Treat. 2016; 2016:8340863. PMID: 27022480.
Article31. Christodoulou C, Junghaenel DU, DeWalt DA, Rothrock N, Stone AA. Cognitive interviewing in the evaluation of fatigue items: results from the patient-reported outcomes measurement information system (PROMIS). Qual Life Res. 2008; 17(10):1239–1246. PMID: 18850327.
Article32. Liegl G, Rose M, Correia H, Fischer HF, Kanlidere S, Mierke A, et al. An initial psychometric evaluation of the German PROMIS v1.2 physical function item bank in patients with a wide range of health conditions. Clin Rehabil. 2018; 32(1):84–93. PMID: 28604084.
Article33. Liu Y, Hinds PS, Wang J, Correia H, Du S, Ding J, et al. Translation and linguistic validation of the pediatric patient-reported outcomes measurement information system measures into simplified Chinese using cognitive interviewing methodology. Cancer Nurs. 2013; 36(5):368–376. PMID: 23860394.
Article34. Mahmoud GA, Rady HM, Mostafa AM. Cross cultural adaptation and validation of an Arabic version of selected PROMIS measures for use in rheumatoid arthritis patients. Egypt Rheumatol. 2019; 41(3):177–182.
Article35. Rhee Y, Jun S, Choi SE. Concepts and applications of patient-reported outcomes & quality of life measure: practical recommendations for Korea. J Health Technol Assess. 2015; 3(1):48–58.36. Cho J, Yoon J, Kim Y, Park S. Development and Clinical Validation of Patient-Reported Outcome Measurements for Chronic Disease Patients for Precision Medicine. Cheongju, Korea: Korea Ministry of Food and Drug Safety;2018.37. Oude Voshaar MA, Ten Klooster PM, Taal E, Krishnan E, van de Laar MA. Dutch translation and cross-cultural adaptation of the PROMIS® physical function item bank and cognitive pre-test in Dutch arthritis patients. Arthritis Res Ther. 2012; 14(2):R47. PMID: 22390734.
Article38. Bae JM. Indices for the responsiveness and interpretability in patient-reported outcomes. Korean J Fam Pract. 2015; 5(3):161–166.39. Cho J, Yoon J, Kim Y, Oh D, Kim SJ, Ahn J, et al. Linguistic validation of the US national cancer institute's patient-reported outcomes version of the common terminology criteria for adverse events in Korean. J Glob Oncol. 2019; 5(5):1–10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Validation of the Korean Version of the Patient-Reported Outcomes Information System® Emotional Distress Measures
- Translation and Linguistic Validation of Korean Version of the Expanded Prostate Cancer Index Composite for Clinical Practice for Patients With Prostate Cancer
- Translation and Linguistic Validation of the Korean Version of the Wisconsin Stone Quality of Life Questionnaire
- Translation and Linguistic Validation of the Korean Version of the "Benefit, Satisfaction, and Willingness to Continue" Questionnaire for Patients With Overactive Bladder
- Korean Translation and Linguistic Validation of Urgency and Overactive Bladder Questionnaires