Diabetes Metab J.
2021 Jul;45(4):594-605. 10.4093/dmj.2020.0049.
Role of Autophagy in Granulocyte-Colony Stimulating Factor Induced Anti-Apoptotic Effects in Diabetic Cardiomyopathy
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea
- 2Department of Cardiology, Jilin University Jilin Central Hospital, Jilin, China
- 3Graguate School of Biomedical Science and Engineering, Hanyang University, Seoul, Korea
- 4Laboratory Medicine, Kangwon National University School of Medicine, Chuncheon, Korea
- 5Department of Thoracic Surgery, Hanyang University College of Medicine, Seoul, Korea
- KMID: 2518901
- DOI: http://doi.org/10.4093/dmj.2020.0049
Abstract
- Background
We previously, reported that granulocyte-colony stimulating factor (G-CSF) reduces cardiomyocyte apoptosis in diabetic cardiomyopathy. However, the underlying mechanisms are not yet fully understood. Therefore, we investigated whether the mechanisms underlying of the anti-apoptotic effects of G-CSF were associated with autophagy using a rat model of diabetic cardiomyopathy.
Methods
Diabetic cardiomyopathy was induced in rats through a high-fat diet combined with low-dose streptozotocin and the rats were then treated with G-CSF for 5 days. Rat H9c2 cardiac cells were cultured under high glucose conditions as an in vitro model of diabetic cardiomyopathy. The extent of apoptosis and protein levels related to autophagy (Beclin-1, microtubule-binding protein light chain 3 [LC3]-II/LC3-I ratio, and P62) were determined for both models. Autophagy determination was performed using an Autophagy Detection kit.
Results
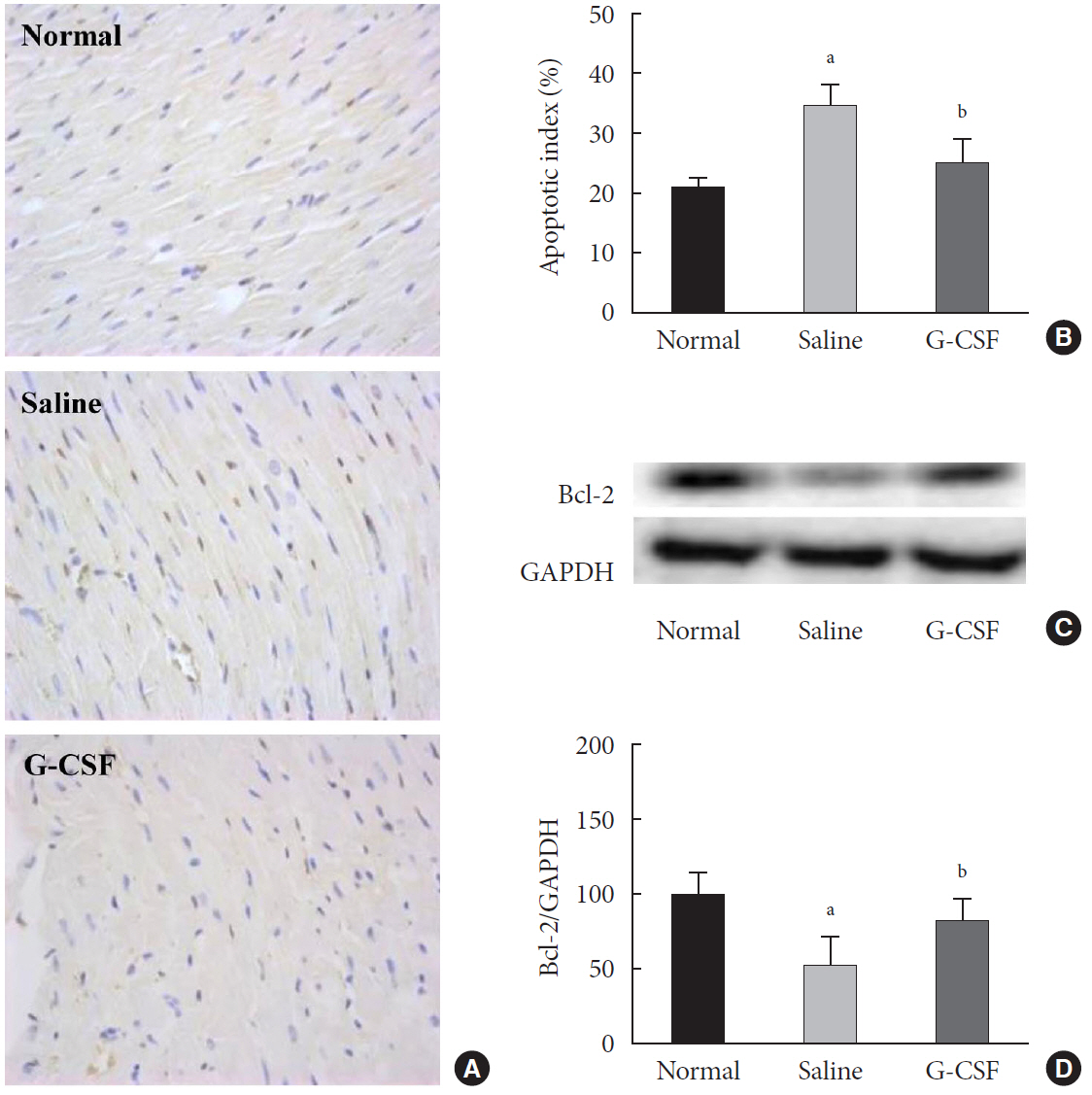
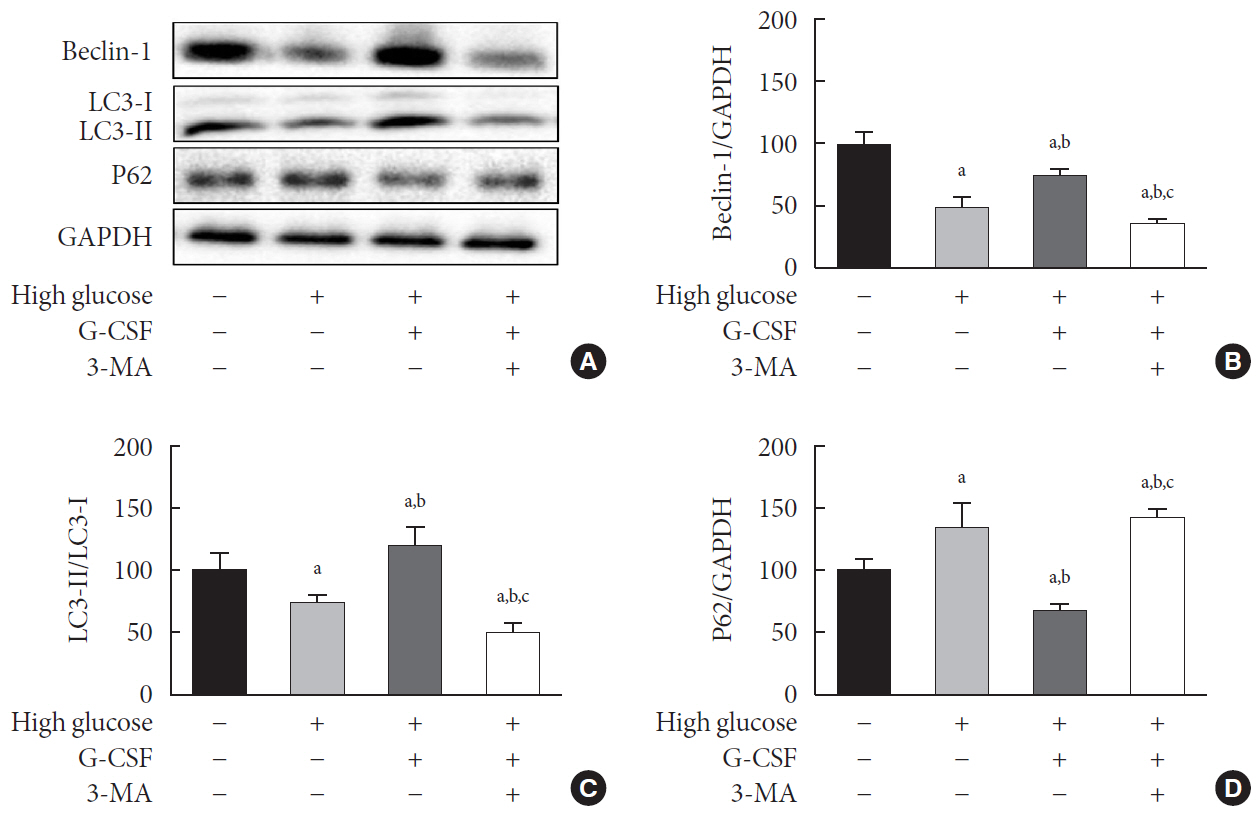
G-CSF significantly reduced cardiomyocyte apoptosis in the diabetic myocardium in vivo and led to an increase in Beclin-1 level and the LC3-II/LC3-I ratio, and decreased P62 level. Similarly, G-CSF suppressed apoptosis, increased Beclin-1 level and LC3-II/LC3-I ratio, and decreased P62 level in high glucose-induced H9c2 cardiac cells in vitro. These effects of G-CSF were abrogated by 3-methyladenine, an autophagy inhibitor. In addition, G-CSF significantly increased autophagic flux in vitro.
Conclusion
Our results suggest that the anti-apoptotic effect of G-CSF might be significantly associated with the up-regulation of autophagy in diabetic cardiomyopathy.
Keyword
Figure
Reference
-
1. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972; 30:595–602.
Article2. Acar E, Ural D, Bildirici U, Sahin T, Yilmaz I. Diabetic cardiomyopathy. Anadolu Kardiyol Derg. 2011; 11:732–7.
Article3. Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemiainduced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002; 51:1938–48.
Article4. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, et al. Myocardial cell death in human diabetes. Circ Res. 2000; 87:1123–32.
Article5. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010; 221:3–12.
Article6. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008; 132:27–42.
Article7. Orogo AM, Gustafsson AB. Therapeutic targeting of autophagy: potential and concerns in treating cardiovascular disease. Circ Res. 2015; 116:489–503.8. Zou MH, Xie Z. Regulation of interplay between autophagy and apoptosis in the diabetic heart: new role of AMPK. Autophagy. 2013; 9:624–5.9. Hsu HC, Chen CY, Lee BC, Chen MF. High-fat diet induces cardiomyocyte apoptosis via the inhibition of autophagy. Eur J Nutr. 2016; 55:2245–54.
Article10. Mellor KM, Reichelt ME, Delbridge LM. Autophagy anomalies in the diabetic myocardium. Autophagy. 2011; 7:1263–7.
Article11. Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991; 78:2791–808.
Article12. Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, et al. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006; 20:956–8.
Article13. Huttmann A, Duhrsen U, Stypmann J, Noppeney R, Nuckel H, Neumann T, et al. Granulocyte colony-stimulating factor-induced blood stem cell mobilisation in patients with chronic heart failure: feasibility, safety and effects on exercise tolerance and cardiac function. Basic Res Cardiol. 2006; 101:78–86.14. Lim YH, Joe JH, Jang KS, Song YS, So BI, Fang CH, et al. Effects of granulocyte-colony stimulating factor (G-CSF) on diabetic cardiomyopathy in Otsuka Long-Evans Tokushima fatty rats. Cardiovasc Diabetol. 2011; 10:92.
Article15. Shin JH, Lim YH, Song YS, So BI, Park JY, Fang CH, et al. Granulocyte-colony stimulating factor reduces cardiomyocyte apoptosis and ameliorates diastolic dysfunction in Otsuka Long-Evans Tokushima Fatty rats. Cardiovasc Drugs Ther. 2014; 28:211–20.
Article16. Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocintreated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005; 52:313–20.
Article17. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8:e1000412.
Article18. Ti Y, Xie GL, Wang ZH, Bi XL, Ding WY, Wang J, et al. TRB3 gene silencing alleviates diabetic cardiomyopathy in a type 2 diabetic rat model. Diabetes. 2011; 60:2963–74.
Article19. Song YS, Fang CH, So BI, Park JY, Lee Y, Shin JH, et al. Time course of the development of nonalcoholic Fatty liver disease in the Otsuka long-evans Tokushima Fatty rat. Gastroenterol Res Pract. 2013; 2013:342648.
Article20. Song YS, Joo HW, Park IH, Shen GY, Lee Y, Shin JH, et al. Transplanted human amniotic epithelial cells secrete paracrine proangiogenic cytokines in rat model of myocardial infarction. Cell Transplant. 2015; 24:2055–64.21. Liu L, Ding WY, Zhao J, Wang ZH, Zhong M, Zhang W, et al. Activin receptor-like kinase 7 mediates high glucose-induced H9c2 cardiomyoblast apoptosis through activation of Smad2/3. Int J Biochem Cell Biol. 2013; 45:2027–35.
Article22. Song YS, Joo HW, Park IH, Shen GY, Lee Y, Shin JH, et al. Bone marrow mesenchymal stem cell-derived vascular endothelial growth factor attenuates cardiac apoptosis via regulation of cardiac miRNA-23a and miRNA-92a in a rat model of myocardial infarction. PLoS One. 2017; 12:e0179972.
Article23. Sharif T, Martell E, Dai C, Kennedy BE, Murphy P, Clements DR, et al. Autophagic homeostasis is required for the pluripotency of cancer stem cells. Autophagy. 2017; 13:264–84.
Article24. Almasi S, Kennedy BE, El-Aghil M, Sterea AM, Gujar S, Partida-Sanchez S, et al. TRPM2 channel-mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway. J Biol Chem. 2018; 293:3637–50.
Article25. Perez-Arizti JA, Ventura-Gallegos JL, Galvan Juarez RE, Ramos-Godinez MDP, Colin-Val Z, Lopez-Marure R. Titanium dioxide nanoparticles promote oxidative stress, autophagy and reduce NLRP3 in primary rat astrocytes. Chem Biol Interact. 2020; 317:108966.26. Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004; 25:543–67.
Article27. Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014; 142:375–415.
Article28. Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, et al. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II-dependent. Lab Invest. 2000; 80:513–27.29. Li K, Cui YC, Zhang H, Liu XP, Zhang D, Wu AL, et al. Glutamine reduces the apoptosis of H9C2 cells treated with high-glucose and reperfusion through an oxidation-related mechanism. PLoS One. 2015; 10:e0132402.
Article30. Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest. 2015; 125:55–64.
Article31. Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005; 102:13807–12.
Article32. Zhu H, Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Methods Enzymol. 2009; 453:343–63.33. Sishi BJ, Loos B, van Rooyen J, Engelbrecht AM. Autophagy upregulation promotes survival and attenuates doxorubic-ininduced cardiotoxicity. Biochem Pharmacol. 2013; 85:124–34.
Article34. Wang ZV, Rothermel BA, Hill JA. Autophagy in hypertensive heart disease. J Biol Chem. 2010; 285:8509–14.
Article35. Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol. 2003; 3:219–28.
Article36. Xu X, Hua Y, Nair S, Zhang Y, Ren J. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. J Mol Cell Biol. 2013; 5:61–3.
Article37. Xu X, Kobayashi S, Chen K, Timm D, Volden P, Huang Y, et al. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem. 2013; 288:18077–92.
Article38. Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011; 18:571–80.
Article39. Kobayashi S, Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta. 2015; 1852:252–61.
Article40. Cherra SJ 3rd, Kulich SM, Uechi G, Balasubramani M, Mountzouris J, Day BW, et al. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol. 2010; Aug. 190:533–9.
Article41. Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009; 452:181–97.42. Zhao Y, Zhang L, Qiao Y, Zhou X, Wu G, Wang L, et al. Heme oxygenase-1 prevents cardiac dysfunction in streptozotocin-diabetic mice by reducing inflammation, oxidative stress, apoptosis and enhancing autophagy. PLoS One. 2013; 8:e75927.
Article43. Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011; 60:1770–8.
Article44. Song H, Zandstra PW, Radisic M. Engineered heart tissue model of diabetic myocardium. Tissue Eng Part A. 2011; 17:1869–78.
Article45. Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976; 98:367–81.
Article46. Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991; 69:1476–86.
Article47. Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim. 2011; 47:125–31.48. Jia Z, Liu Y, Su H, Li M, Zhang M, Zhu Y, et al. Safflower extract inhibiting apoptosis by inducing autophagy in myocardium derived H9C2 cell. Int J Clin Exp Med. 2015; 8:20254–62.49. Gao H, Hou F, Dong R, Wang Z, Zhao C, Tang W, et al. RhoKinase inhibitor fasudil suppresses high glucose-induced H9c2 cell apoptosis through activation of autophagy. Cardiovasc Ther. 2016; 34:352–9.
Article50. Guo Y, Liu S, Zhang X, Wang L, Gao J, Han A, et al. G-CSF promotes autophagy and reduces neural tissue damage after spinal cord injury in mice. Lab Invest. 2015; 95:1439–49.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of MicroRNA-34a in Anti-Apoptotic Effects of Granulocyte-Colony Stimulating Factor in Diabetic Cardiomyopathy
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- The effect of granulocyte colony-stimulating factor in chemotherapy of acute myelogenous leukemia
- The effects on the production of platelet activating factor in the cultured human endothelial cells by interleukin-6 and granulocyte macrophage colony stimulating factor