Ewha Med J.
2021 Jul;44(3):63-69. 10.12771/emj.2021.44.3.63.
Clinical Characteristics, Treatment Delivery, and Cisplatin Eligibility in Korean Patients Initially Diagnosed with Urothelial Carcinoma
- Affiliations
-
- 1Medical Oncology and Hematology, Department of Internal Medicine, Pusan National University Yangsan Hospital, Korea
- 2Departments of Urology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 3Departments of Pathology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 4Departments of Radiology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 5Department of Neurology, Uijeongbu St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- KMID: 2518768
- DOI: http://doi.org/10.12771/emj.2021.44.3.63
Abstract
Objectives
The aim of this study was to examine the clinical presentation, treatment delivery, and cisplatin eligibility of Korean patients with urothelial carcinoma (UC) in a real-world setting.
Methods
We performed a retrospective cohort study of patients initially diagnosed with UC from March 2013 to June 2018. Creatinine clearance >60 mL/min and Eastern Cooperative Oncology Group performance status (0–1) were adopted as cisplatin eligibility criteria.
Results
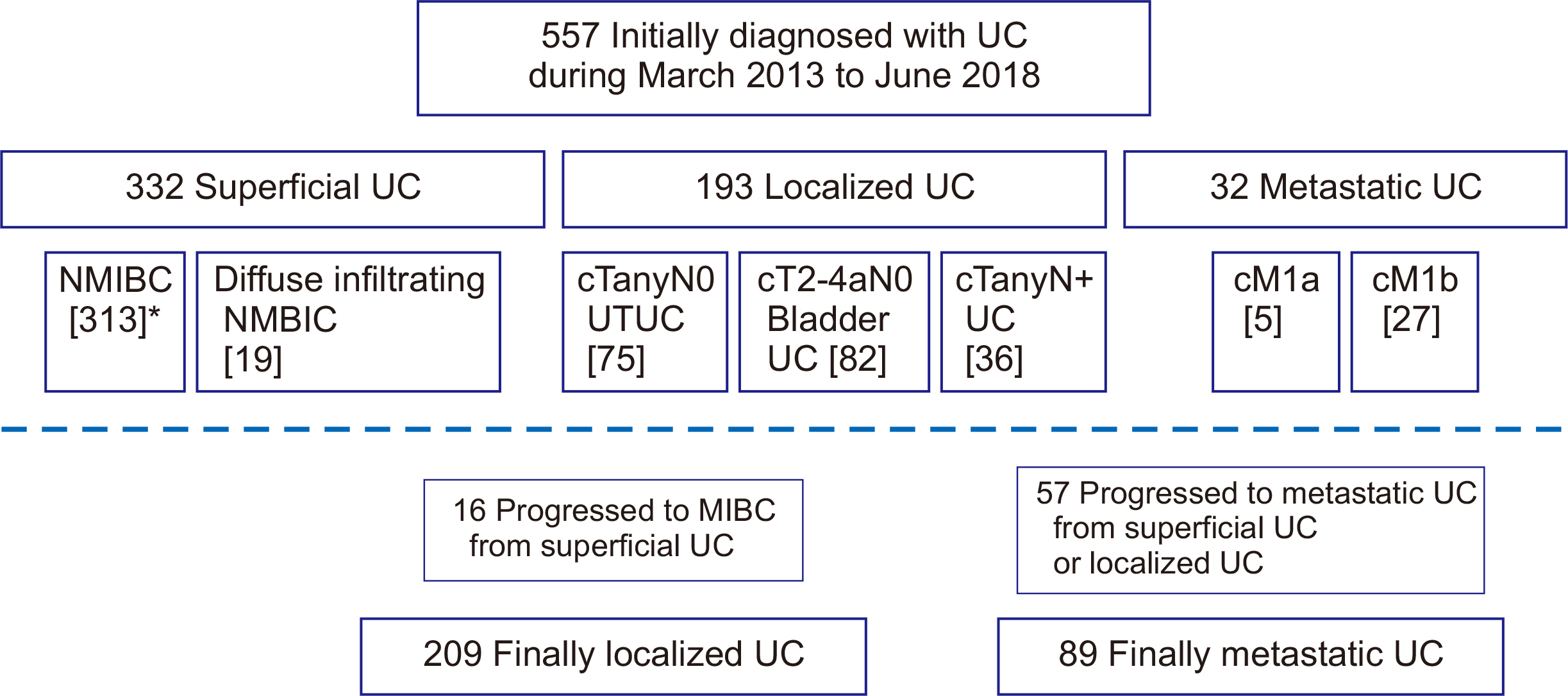
This study included 557 eligible patients. Median age was 71.0 years (range, 33–94 years), and males were dominant (80%). Primary tumor sites were: upper genitourinary tract, 18%; bladder, 81%; and urethra, 0.4%. Initial disease status was non-muscle invasive bladder cancer (313, 56%), diffuse infiltrating non-muscle invasive bladder cancer (19, 3%), cTanyN0 upper tract UC (75, 13%), cT2-4N0 bladder UC (82, 15%), TanyN1-3 UC (36, 7%), or initially metastatic UC (32, 6%). At the time of analysis (June 2019), following treatments were delivered to 134 patients with localized UC: radical operation with or without perioperative treatment (89, 67%), definitive chemoradiation (7, 5%), and palliative surgery or supportive care only (36, 28%). In total, 89 patients had metastatic UC, including those with recurrent disease (n=57), and 34 (38%) of the 89 were eligible for cisplatin.
Conclusion
Clinical presentations in East Asian UC patients were consistent with those of previous studies in other countries, except for a relatively high incidence of upper genitourinary tract. Our results can serve as a benchmark for further advances and future research for treatments of UC in East Asian patients.
Keyword
Figure
Reference
-
1. Bach PB, Cramer LD, Warren JL, Begg CB. 1999; Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 341:1198–1205. DOI: 10.1056/NEJM199910143411606. PMID: 10519898.
Article2. Robinson AG, Wei X, Mackillop WJ, Peng Y, Booth CM. 2016; Use of palliative chemotherapy for advanced bladder cancer: patterns of care in routine clinical practice. J Natl Compr Canc Netw. 14:291–298. DOI: 10.6004/jnccn.2016.0034. PMID: 26957615.
Article3. Snyder C, Harlan L, Knopf K, Potosky A, Kaplan R. 2003; Patterns of care for the treatment of bladder cancer. J Urol. 169:1697–1701. DOI: 10.1097/01.ju.0000056727.30546.b7. PMID: 12686811.
Article4. Robinson AG, Wei X, Vera-Badillo FE, Mackillop WJ, Booth CM. 2017; Palliative chemotherapy for bladder cancer: treatment delivery and outcomes in the general population. Clin Genitourin Cancer. 15:e535–e541. DOI: 10.1016/j.clgc.2016.12.025. PMID: 28117134.
Article5. Bamias A, Peroukidis S, Stamatopoulou S, Tzannis K, Koutsoukos K, Andreadis C, et al. 2016; Utilization of systemic chemotherapy in advanced urothelial cancer: a retrospective collaborative study by the Hellenic Genitourinary Cancer Group (HGUCG). Clin Genitourin Cancer. 14:e153–e159. DOI: 10.1016/j.clgc.2015.09.009. PMID: 26437909.
Article6. Wu YT, Luo HL, Wang HJ, Chen YT, Cheng YT, Chiang PH. 2020; Gender effect on the oncologic outcomes of upper urinary tract urothelial carcinoma in Taiwan. Int Urol Nephrol. 52:1043–1048. DOI: 10.1007/s11255-020-02396-z. PMID: 31997062.
Article7. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. 2018; European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 73:111–122. DOI: 10.1016/j.eururo.2017.07.036. PMID: 28867446.8. Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. 2009; Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 115:1224–1233. DOI: 10.1002/cncr.24135. PMID: 19156917.
Article9. Hussain MH, Wood DP, Bajorin DF, Bochner BH, Dreicer R, Lamm DL, et al. 2009; Bladder cancer: narrowing the gap between evidence and practice. J Clin Oncol. 27:5680–5684. DOI: 10.1200/JCO.2009.23.6901. PMID: 19858384. PMCID: PMC4659855.
Article10. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. 2005; Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 23:4602–4608. DOI: 10.1200/JCO.2005.07.757. PMID: 16034041.
Article11. Bamias A, Tzannis K, Harshman LC, Crabb SJ, Wong YN, Kumar Pal S, et al. 2018; Impact of contemporary patterns of chemotherapy utilization on survival in patients with advanced cancer of the urinary tract: a Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC). Ann Oncol. 29:361–369. DOI: 10.1093/annonc/mdx692. PMID: 29077785. PMCID: PMC7360142.
Article12. Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. 2003; Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 349:859–866. DOI: 10.1056/NEJMoa022148. PMID: 12944571.
Article13. International Collaboration of Trialists. 1999; Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. Lancet. 354:533–540. DOI: 10.1016/S0140-6736(99)02292-8. PMID: 10470696.14. Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. 2011; Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J Clin Oncol. 29:2432–2438. DOI: 10.1200/JCO.2011.34.8433. PMID: 21555688.
Article15. Browne BM, Stensland KD, Moynihan MJ, Canes D. 2018; An analysis of staging and treatment trends for upper tract urothelial carcinoma in the National Cancer Database. Clin Genitourin Cancer. 16:e743–e750. DOI: 10.1016/j.clgc.2018.01.015. PMID: 29506950.
Article16. Birtle AJ, Chester JD, Jones RJ, Johnson M, Hill M, Bryan RT, et al. 2018; Results of POUT: a phase III randomised trial of perioperative chemotherapy versus surveillance in upper tract urothelial cancer (UTUC). J Clin Oncol. 36(6 Suppl):407. DOI: 10.1200/JCO.2018.36.6_suppl.407.
Article17. Donat SM. 2009; Integrating perioperative chemotherapy into the treatment of muscle-invasive bladder cancer: strategy versus reality. J Natl Compr Canc Netw. 7:40–47. DOI: 10.6004/jnccn.2009.0003. PMID: 19176204.
Article18. Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. 2005; Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 48:202–206. DOI: 10.1016/j.eururo.2005.04.006. PMID: 15939524.19. Kaag MG, O'Malley RL, O'Malley P, Godoy G, Chen M, Smaldone MC, et al. 2010; Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 58:581–587. DOI: 10.1016/j.eururo.2010.06.029. PMID: 20619530. PMCID: PMC3677959.
Article20. Donat SM, Shabsigh A, Savage C, Cronin AM, Bochner BH, Dalbagni G, et al. 2009; Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol. 55:177–185. DOI: 10.1016/j.eururo.2008.07.018. PMID: 18640770.
Article21. Sonpavde G, Watson D, Tourtellott M, Cowey CL, Hellerstedt B, Hutson TE, et al. 2012; Administration of cisplatin-based chemotherapy for advanced urothelial carcinoma in the community. Clin Genitourin Cancer. 10:1–5. DOI: 10.1016/j.clgc.2011.11.005. PMID: 22340630.
Article22. Bae WK, Lee HJ, Park SH, Kim JH, Kim HJ, Maeng CH, et al. 2019; Comparative effectiveness of palliative chemotherapy versus neoadjuvant chemotherapy followed by radical cystectomy versus cystectomy followed by adjuvant chemotherapy versus cystectomy for regional node-positive bladder cancer: a retrospective analysis: KCSG GU 17-03. Cancer Med. 8:5431–5437. DOI: 10.1002/cam4.2446. PMID: 31353788. PMCID: PMC6745843.
Article23. Galsky MD, Stensland K, Sfakianos JP, Mehrazin R, Diefenbach M, Mohamed N, et al. 2016; Comparative effectiveness of treatment strategies for bladder cancer with clinical evidence of regional lymph node involvement. J Clin Oncol. 34:2627–2635. DOI: 10.1200/JCO.2016.67.5033. PMID: 27269939. PMCID: PMC5012691.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy in Advanced Urothelial Carcinoma
- M-VAC(Methotrexate, Vinblastine, Doxorubicin and Cisplatin) for Advanced Urothelial Tumors
- Efficacy and Toxicity of Gemcitabine Plus Cisplatin Chemotherapy in Advanced Urothelial Cancer
- The Comparison of the Efficacy and Side Effects between M-VAC and GC Chemotherapy for Advanced or Metastatic Urothelial Carcinoma Patients with a Good Performance Status
- Sarcomatoid urothelial carcinoma arising in the female urethral diverticulum