Cancer Res Treat.
2021 Jul;53(3):611-620. 10.4143/crt.2021.066.
Physical and Biological Characteristics of Particle Therapy for Oncologists
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2518384
- DOI: http://doi.org/10.4143/crt.2021.066
Abstract
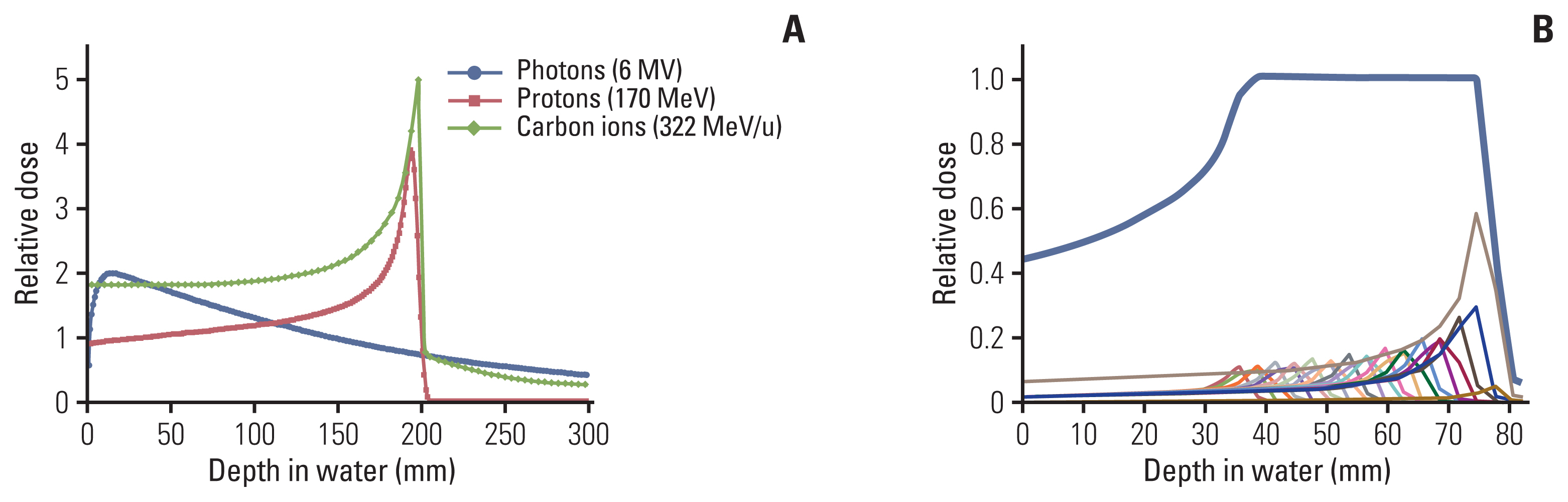
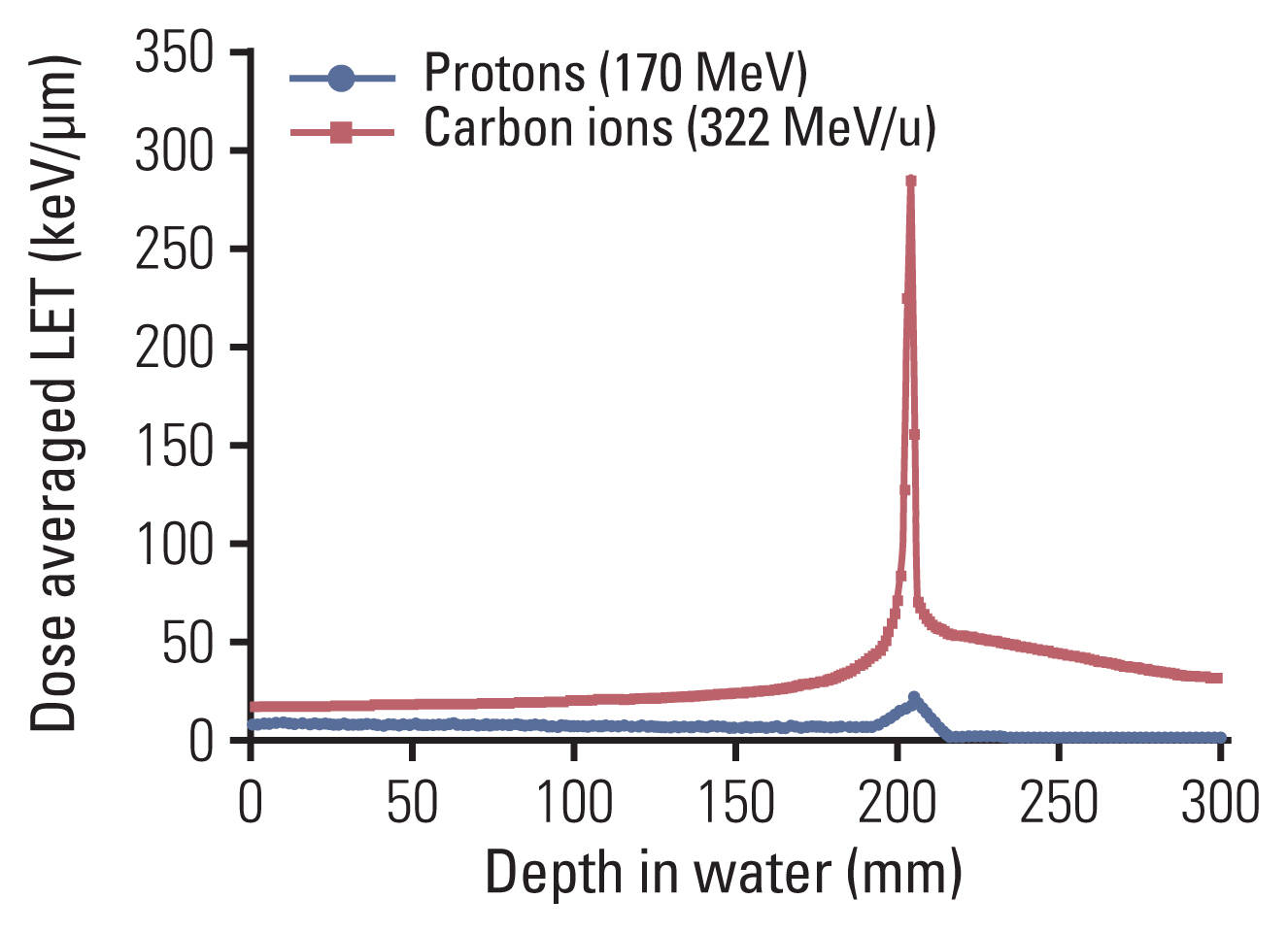
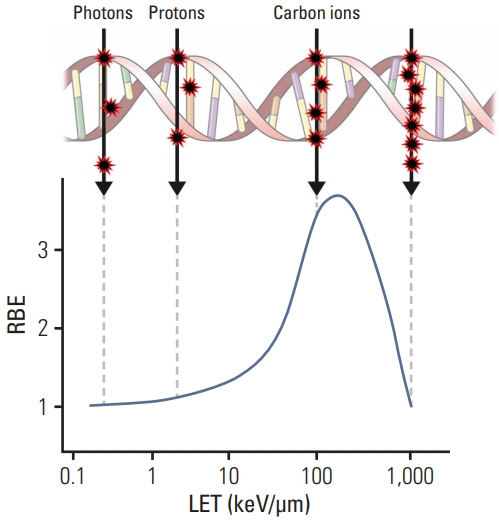
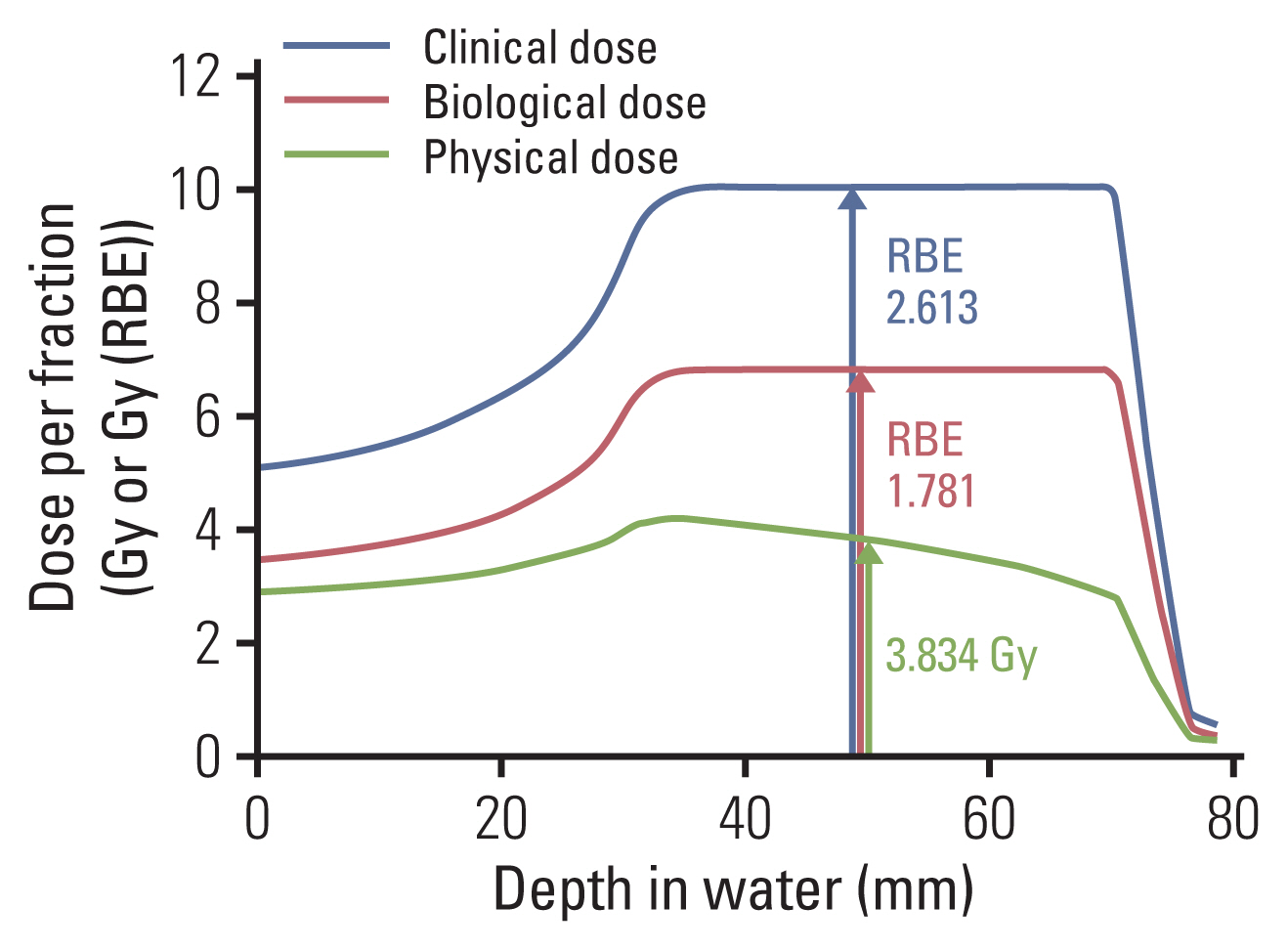
- Particle therapy is a promising and evolving modality of radiotherapy that can be used to treat tumors that are radioresistant to conventional photon beam radiotherapy. It has unique biological and physical advantages compared with conventional radiotherapy. The characteristic feature of particle therapy is the “Bragg peak,” a steep and localized peak of dose, that enables precise delivery of the radiation dose to the tumor while effectively sparing normal organs. Especially, the charged particles (e.g., proton, helium, carbon) cause a high rate of energy loss along the track, thereby leading to high biological effectiveness, which makes particle therapy attractive. Using this property, the particle beam induces more severe DNA double-strand breaks than the photon beam, which is less influenced by the oxygen level. This review describes the general biological and physical aspects of particle therapy for oncologists, including non-radiation oncologists and beginners in the field.
Keyword
Figure
Cited by 2 articles
-
The Rise of Particle Beam Therapy: Are We Ready for The Potential Game-Changer?
Eui Kyu Chie, Yong Chan Ahn
Cancer Res Treat. 2021;53(3):609-610. doi: 10.4143/crt.2021.707.Clinical indications and future directions of carbon-ion radiotherapy: a narrative review
Seo Hee Choi, Woong Sub Koom, Hong In Yoon, Kyung Hwan Kim, Chan Woo Wee, Jaeho Cho, Yong Bae Kim, Ki Chang Keum, Ik Jae Lee
Ewha Med J. 2024;47(4):e56. doi: 10.12771/emj.2024.e56.
Reference
-
References
1. Halperin EC. Particle therapy and treatment of cancer. Lancet Oncol. 2006; 7:676–85.
Article2. Slater JM, Archambeau JO, Miller DW, Notarus MI, Preston W, Slater JD. The proton treatment center at Loma Linda University Medical Center: rationale for and description of its development. Int J Radiat Oncol Biol Phys. 1992; 22:383–9.
Article3. Tsuji H, Akine Y, Okumura T. Proton radiotherapy: clinical experience and outcome at Tsukuba. Radiol Nuclear Med. 1997; 43:604–9.4. Schaub L, Harrabi SB, Debus J. Particle therapy in the future of precision therapy. Br J Radiol. 2020; 93:20200183.
Article5. Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014; 15:1027–38.
Article6. Particle therapy facilities in clinical operation (last update Feb 2021) [Internet]. Villigen: Particle Therapy Co-Operative Group;2021. [cited 2021 Jun 14]. Available from: https://ptcog.ch/index.php/facilities-in-operation .7. Particle therapy facilities under construction (last update Feb 2021) [Internet]. Villigen: Particle Therapy Co-Operative Group;2021. [cited 2021 Jun 14]. Available from: https://ptcog.ch/index.php/facilities-under-construction .8. Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014; 36:1747–53.
Article9. Mohan R, Liu AY, Brown PD, Mahajan A, Dinh J, Chung C, et al. Proton therapy reduces the likelihood of high-grade radiation-induced lymphopenia in glioblastoma patients: phase II randomized study of protons vs photons. Neuro Oncol. 2021; 23:284–94.
Article10. Routman DM, Garant A, Lester SC, Day CN, Harmsen WS, Sanheuza CT, et al. A comparison of grade 4 lymphopenia with proton versus photon radiation therapy for esophageal cancer. Adv Radiat Oncol. 2019; 4:63–9.
Article11. Xiang M, Chang DT, Pollom EL. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer. 2020; 126:3560–8.
Article12. Mohamad O, Tabuchi T, Nitta Y, Nomoto A, Sato A, Kasuya G, et al. Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: a propensity score-weighted, retrospective, cohort study. Lancet Oncol. 2019; 20:674–85.
Article13. Saunders W, Castro JR, Chen GT, Collier JM, Zink SR, Pitluck S, et al. Helium-ion radiation therapy at the Lawrence Berkeley Laboratory: recent results of a Northern California Oncology Group Clinical Trial. Radiat Res Suppl. 1985; 8:S227–34.
Article14. Tessonnier T, Mairani A, Brons S, Sala P, Cerutti F, Ferrari A, et al. Helium ions at the heidelberg ion beam therapy center: comparisons between FLUKA Monte Carlo code predictions and dosimetric measurements. Phys Med Biol. 2017; 62:6784–803.
Article15. Tobias CA, Anger HO, Lawrence JH. Radiological use of high energy deuterons and alpha particles. Am J Roentgenol Radium Ther Nucl Med. 1952; 67:1–27.16. Lawrence JH, Tobias CA, Born JL, Mc CR, Roberts JE, Anger HO, et al. Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer Res. 1958; 18:121–34.17. Castro JR, Quivey JM, Lyman JT, Chen GT, Phillips TL, Tobias CA. Radiotherapy with heavy charged particles at Lawrence Berkeley Laboratory. J Can Assoc Radiol. 1980; 31:30–4.18. Chen GT, Castro JR, Quivey JM. Heavy charged particle radiotherapy. Annu Rev Biophys Bioeng. 1981; 10:499–529.
Article19. Chatterjee A, Alpen EL, Tobias CA, Llacer J, Alonso J. High energy beams of radioactive nuclei and their biomedical applications. Int J Radiat Oncol Biol Phys. 1981; 7:503–7.
Article20. Particle Therapy Co-Operative Group [Internet]. Villigen: Particle Therapy Co-Operative Group;2021. [cited 2021 Jun 14]. Available from: https://www.ptcog.ch/ .21. Tsujii H, Kamada T, Shirai T, Noda K, Tsuji H, Karasawa K. Carbon-ion radiotherapy: principles, practices, and treatment planning. Tokyo: Springer;2014.22. Fredriksson A. Robust optimization in radiation therapy. Terlaky T, Anjos MF, Ahmed S, editors. Advances and trends in optimization with engineering applications. Philadelphia, PA: SIAM;2017. p. 1–12.23. Han Y. Current status of proton therapy techniques for lung cancer. Radiat Oncol J. 2019; 37:232–48.
Article24. Seco J, Robertson D, Trofimov A, Paganetti H. Breathing interplay effects during proton beam scanning: simulation and statistical analysis. Phys Med Biol. 2009; 54:N283–94.
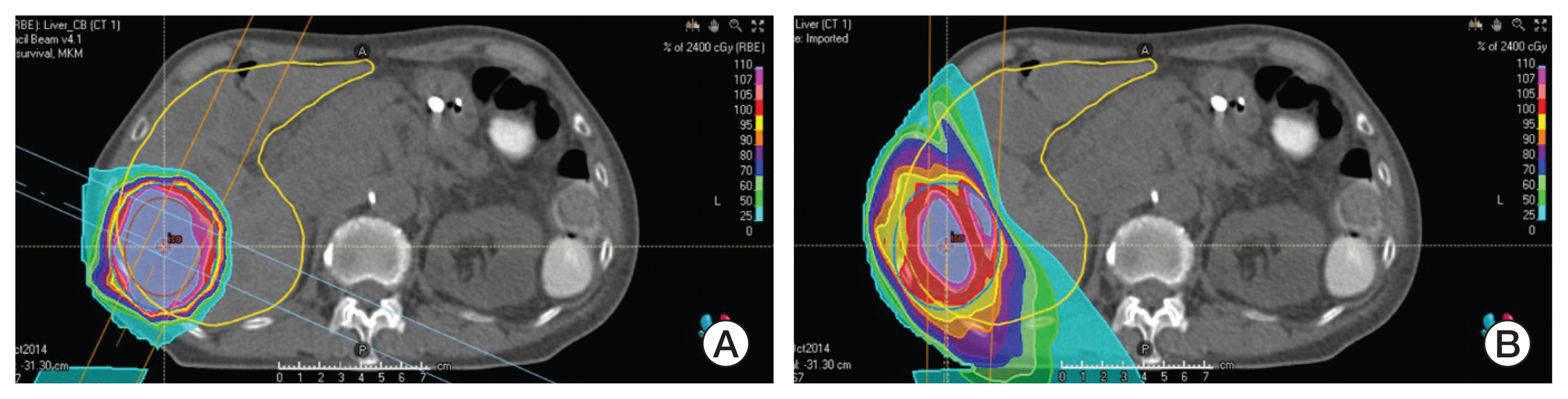
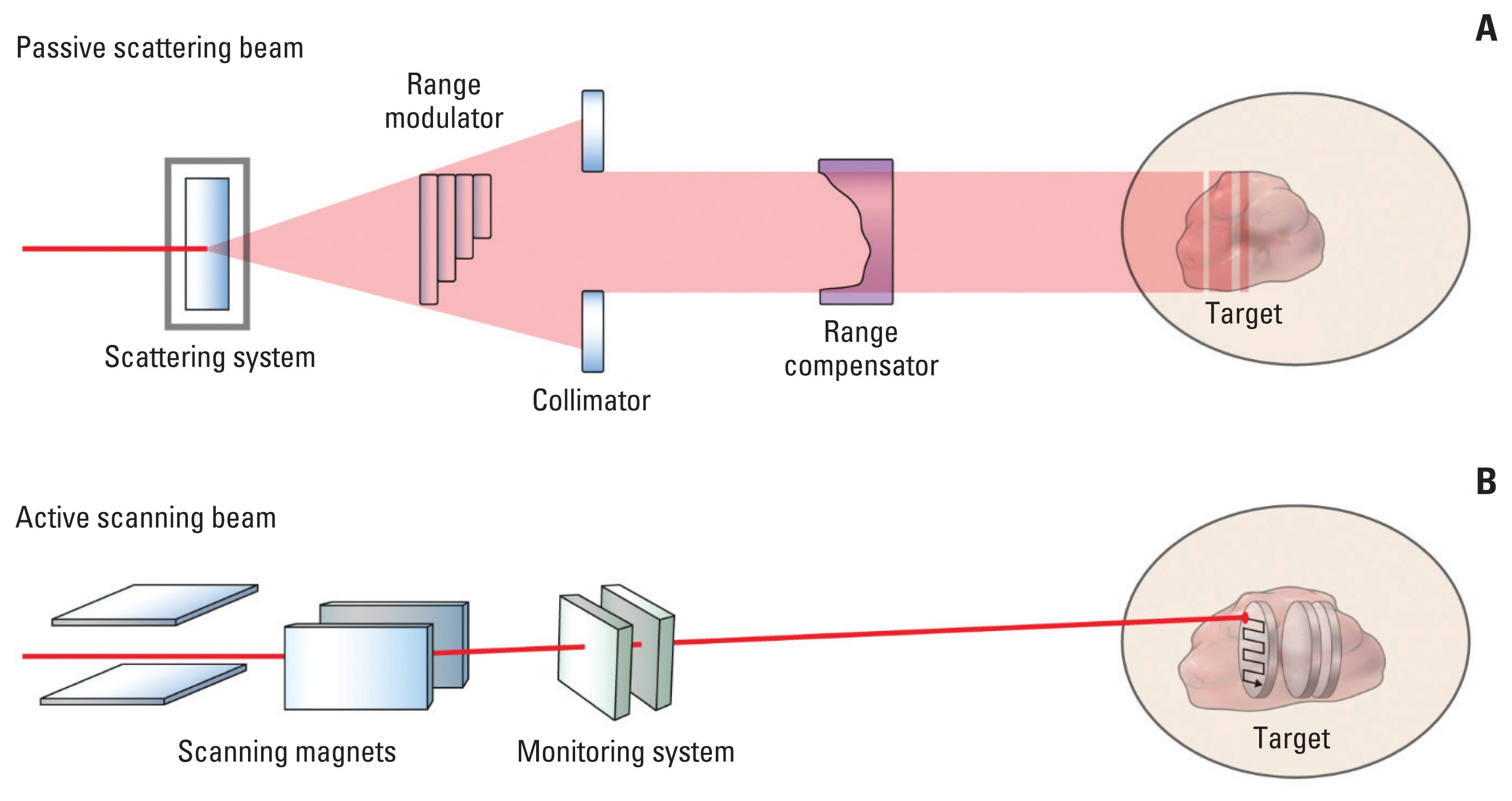
Article25. Yoo GS, Yu JI, Cho S, Jung SH, Han Y, Park S, et al. Comparison of clinical outcomes between passive scattering versus pencil-beam scanning proton beam therapy for hepatocellular carcinoma. Radiother Oncol. 2020; 146:187–93.
Article26. Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7th ed. Philadelphia, PA: Lippincott Williams & Wolters Kluwer;2010. p. 394–6.27. Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002; 53:407–21.
Article28. Choi C, Son A, Lee GH, Shin SW, Park S, Ahn SH, et al. Targeting DNA-dependent protein kinase sensitizes hepatocellular carcinoma cells to proton beam irradiation through apoptosis induction. PLoS One. 2019; 14:e0218049.
Article29. Jones B. Towards achieving the full clinical potential of proton therapy by inclusion of LET and RBE models. Cancers (Basel). 2015; 7:460–80.
Article30. Paganetti H, Blakely E, Carabe-Fernandez A, Carlson DJ, Das IJ, Dong L, et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med Phys. 2019; 46:e53–78.
Article31. Bristow RG, Hill RP. Hypoxia and metabolism: hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008; 8:180–92.32. Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004; 4:437–47.
Article33. West C, Rosenstein BS, Alsner J, Azria D, Barnett G, Begg A, et al. Establishment of a radiogenomics consortium. Int J Radiat Oncol Biol Phys. 2010; 76:1295–6.
Article34. Fachal L, Gomez-Caamano A, Barnett GC, Peleteiro P, Carballo AM, Calvo-Crespo P, et al. A three-stage genome-wide association study identifies a susceptibility locus for late radiotherapy toxicity at 2q24.1. Nat Genet. 2014; 46:891–4.
Article35. Kerns SL, Dorling L, Fachal L, Bentzen S, Pharoah PD, Barnes DR, et al. Meta-analysis of genome wide association studies identifies genetic markers of late toxicity following radiotherapy for prostate cancer. EBio Medicine. 2016; 10:150–63.
Article36. Tinganelli W, Durante M. Carbon ion radiobiology. Cancers (Basel). 2020; 12:3022.
Article37. West C. Translational radiobiology: radiogenomics. In : Proceedings of the PTCOG-58; 2019 Jun 10–15; Manchester, UK.38. Akino Y, Teshima T, Kihara A, Kodera-Suzumoto Y, Inaoka M, Higashiyama S, et al. Carbon-ion beam irradiation effectively suppresses migration and invasion of human non-small-cell lung cancer cells. Int J Radiat Oncol Biol Phys. 2009; 75:475–81.
Article39. Kamlah F, Hanze J, Arenz A, Seay U, Hasan D, Juricko J, et al. Comparison of the effects of carbon ion and photon irradiation on the angiogenic response in human lung adenocarcinoma cells. Int J Radiat Oncol Biol Phys. 2011; 80:1541–9.
Article40. Moncharmont C, Levy A, Guy JB, Falk AT, Guilbert M, Trone JC, et al. Radiation-enhanced cell migration/invasion process: a review. Crit Rev Oncol Hematol. 2014; 92:133–42.
Article41. Sonveaux P, Brouet A, Havaux X, Gregoire V, Dessy C, Balligand JL, et al. Irradiation-induced angiogenesis through the up-regulation of the nitric oxide pathway: implications for tumor radiotherapy. Cancer Res. 2003; 63:1012–9.42. Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res. 2001; 61:2744–50.43. Ogata T, Teshima T, Kagawa K, Hishikawa Y, Takahashi Y, Kawaguchi A, et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res. 2005; 65:113–20.44. Takahashi Y, Teshima T, Kawaguchi N, Hamada Y, Mori S, Madachi A, et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res. 2003; 63:4253–7.45. Subtil FS, Wilhelm J, Bill V, Westholt N, Rudolph S, Fischer J, et al. Carbon ion radiotherapy of human lung cancer attenuates HIF-1 signaling and acts with considerably enhanced therapeutic efficiency. FASEB J. 2014; 28:1412–21.
Article46. Sharif S, Ferner R, Birch JM, Gillespie JE, Gattamaneni HR, Baser ME, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006; 24:2570–5.
Article47. Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016; 17:1558–68.
Article48. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017; 377:1345–56.49. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015; 372:320–30.50. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015; 372:2521–32.
Article51. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015; 1:1325–32.
Article52. Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys. 2016; 95:120–30.
Article53. Bouquet F, Muller C, Salles B. The loss of gamma H2AX signal is a marker of DNA double strand breaks repair only at low levels of DNA damage. Cell Cycle. 2006; 5:1116–22.54. Sato H, Niimi A, Yasuhara T, Permata TB, Hagiwara Y, Isono M, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017; 8:1751.
Article55. Schmid TE, Multhoff G. Non-targeted effects of photon and particle irradiation and the interaction with the immune system. Front Oncol. 2012; 2:80.
Article56. Matsunaga A, Ueda Y, Yamada S, Harada Y, Shimada H, Hasegawa M, et al. Carbon-ion beam treatment induces systemic antitumor immunity against murine squamous cell carcinoma. Cancer. 2010; 116:3740–8.
Article57. Ando K, Fujita H, Hosoi A, Ma L, Wakatsuki M, Seino KI, et al. Intravenous dendritic cell administration enhances suppression of lung metastasis induced by carbon-ion irradiation. J Radiat Res. 2017; 58:446–55.
Article