J Korean Med Sci.
2021 Jul;36(26):e174. 10.3346/jkms.2021.36.e174.
Additional Drug Resistance in Patients with Multidrug-resistant Tuberculosis in Korea: a Multicenter Study from 2010 to 2019
- Affiliations
-
- 1Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 2Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea
- 3Department of Internal Medicine, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 4Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- 5Division of Pulmonary, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Busan, Korea
- 6Department of Internal Medicine, Dong-A University Hospital, Busan, Korea
- 7Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 8Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- KMID: 2518007
- DOI: http://doi.org/10.3346/jkms.2021.36.e174
Abstract
- Background
Drug-resistance surveillance (DRS) data provide key information for building an effective treatment regimen in patients with multidrug-resistant tuberculosis (MDR-TB). This study was conducted to investigate the patterns and trends of additional drug resistance in MDR-TB patients in South Korea.
Methods
Phenotypic drug susceptibility test (DST) results of MDR-TB patients collected from seven hospitals in South Korea from 2010 to 2019 were retrospectively analyzed.
Results
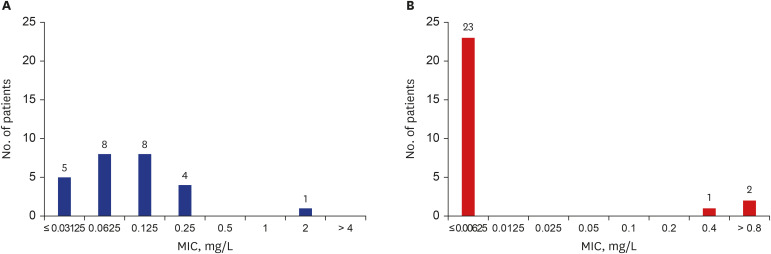
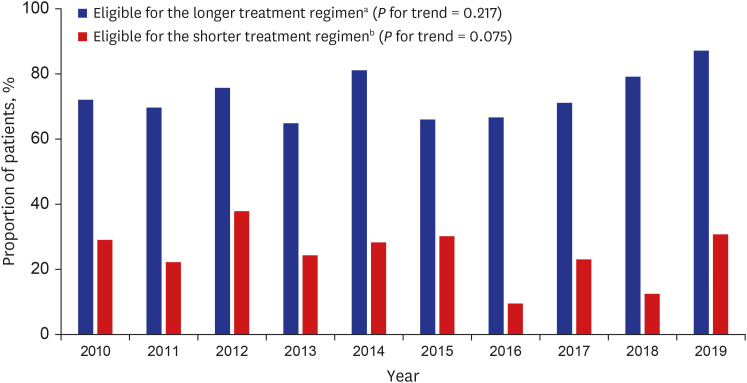
In total, 633 patients with MDR-TB were included in the analysis. Of all patients, 361 (57.0%) were new patients. All patients had additional resistance to a median of three antiTB drugs. The resistance rates of any fluoroquinolone (FQ), linezolid, and cycloserine were 26.2%, 0.0%, and 6.3%, respectively. The proportions of new patients and resistance rates of most anti-TB drugs did not decrease during the study period. The number of additional resistant drugs was significantly higher in FQ-resistant MDR-TB than in FQ-susceptible MDR-TB (median of 9.0 vs. 2.0). Among 26 patients with results of minimum inhibitory concentrations for bedaquiline (BDQ) and delamanid (DLM), one (3.8%) and three (11.5%) patients were considered resistant to BDQ and DLM with interim critical concentrations, respectively. Based on the DST results, 72.4% and 24.8% of patients were eligible for the World Health Organization's longer and shorter MDR-TB treatment regimen, respectively.
Conclusion
The proportions of new patients and rates of additional drug resistance in patients with MDR-TB were high and remain stable in South Korea. A nationwide analysis of DRS data is required to provide effective treatment for MDR-TB patients in South Korea.
Keyword
Figure
Reference
-
1. World Health Organization. Global tuberculosis report 2020. Updated 2020. Accessed March 6, 2021. https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf.2. Lange C, Abubakar I, Alffenaar JWC, Bothamley G, Caminero JA, Carvalho ACC, et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J. 2014; 44(1):23–63. PMID: 24659544.
Article3. Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, et al. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med. 2014; 2(4):321–338. PMID: 24717628.
Article4. Yang C, Luo T, Shen X, Wu J, Gan M, Xu P, et al. Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: a retrospective observational study using whole-genome sequencing and epidemiological investigation. Lancet Infect Dis. 2017; 17(3):275–284. PMID: 27919643.5. World Health Organization. WHO operational handbook on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. Updated 2020. Accessed March 6, 2021. https://www.who.int/publications/i/item/9789240006997.6. Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994–2004. Int J Tuberc Lung Dis. 2007; 11(5):571–576. PMID: 17439684.7. Choi JC, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Drug resistance rates of Mycobacterium tuberculosis at a private referral center in Korea. J Korean Med Sci. 2007; 22(4):677–681. PMID: 17728509.8. Lee HY, Lee J, Lee YS, Kim MY, Lee HK, Lee YM, et al. Drug-resistance pattern of Mycobacterium tuberculosis strains from patients with pulmonary and extrapulmonary tuberculosis during 2006 to 2013 in a Korean tertiary medical center. Korean J Intern Med. 2015; 30(3):325–334. PMID: 25995663.9. Kim SY, Kim HJ, Kim CK, Yoon HR, Bae HG, Lee SH, et al. The recent status of multidrug-and extensively drug-resistant tuberculosis in Korea. Tuberc Respir Dis (Seoul). 2010; 68(3):146–154.10. Mok JH, Kang BH, Lee T, Lee HK, Jang HJ, Cho YJ, et al. Additional drug resistance patterns among multidrug-resistant tuberculosis patients in Korea: implications for regimen design. J Korean Med Sci. 2017; 32(4):636–641. PMID: 28244290.
Article11. World Health Organization. Definitions and reporting framework for tuberculosis - 2013 revision. Updated 2020. Accessed March 6, 2021. https://apps.who.int/iris/bitstream/handle/10665/79199/9789241505345_eng.pdf?sequence=1.12. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. Updated 2020. Accessed March 6, 2021. https://www.who.int/publications/i/item/9789240007048.13. World Health Organization. Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. Updated 2018. Accessed March 6, 2021. https://www.who.int/publications/i/item/WHO-CDS-TB-2018.5.14. European Committee on Antimicrobial Susceptibility Testing. Rationale for EUCAST clinical breakpoints: bedaquiline (version 1.3). Updated 2019. Accessed March 6, 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/190704_Bedaquiline_rational_document.pdf.15. European Committee on Antimicrobial Susceptibility Testing. Rationale for EUCAST clinical breakpoints: delamanid (version 1.2). Updated 2019. Accessed March 6, 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/190704_Delamanid_RationalDocument.pdf.16. Stinson K, Kurepina N, Venter A, Fujiwara M, Kawasaki M, Timm J, et al. MIC of delamanid (OPC-67683) against Mycobacterium tuberculosis clinical isolates and a proposed critical concentration. Antimicrob Agents Chemother. 2016; 60(6):3316–3322. PMID: 26976868.17. World Health Organization. Meeting report of the WHO expert consultation on the definition of extensively drug-resistant tuberculosis, 27–29 October 2020. Updated 2021. Accessed March 6, 2021. https://www.who.int/publications/i/item/meeting-report-of-the-who-expert-consultation-on-the-definition-of-extensively-drug-resistant-tuberculosis.18. Devasia RA, Blackman A, Gebretsadik T, Griffin M, Shintani A, May C, et al. Fluoroquinolone resistance in Mycobacterium tuberculosis: the effect of duration and timing of fluoroquinolone exposure. Am J Respir Crit Care Med. 2009; 180(4):365–370. PMID: 19483111.19. Huang TS, Kunin CM, Lee SSJ, Chen YS, Tu HZ, Liu YC. Trends in fluoroquinolone resistance of Mycobacterium tuberculosis complex in a Taiwanese medical centre: 1995–2003. J Antimicrob Chemother. 2005; 56(6):1058–1062. PMID: 16204341.20. Falzon D, Gandhi N, Migliori GB, Sotgiu G, Cox HS, Holtz TH, et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J. 2013; 42(1):156–168. PMID: 23100499.
Article21. Lee M, Han J, Kim YR, Kwak N, Kim JH, Park O, et al. Multidrug-resistant tuberculosis in South Korea: a retrospective analysis of national registry data in 2011–2015. Int J Tuberc Lung Dis. 2019; 23(7):850–857. PMID: 31439118.
Article22. Yang JS, Kim KJ, Choi H, Lee SH. Delamanid, bedaquiline, and linezolid minimum inhibitory concentration distributions and resistance-related gene mutations in multidrug-resistant and extensively drug-resistant tuberculosis in Korea. Ann Lab Med. 2018; 38(6):563–568. PMID: 30027700.
Article23. Xu J, Wang B, Hu M, Huo F, Guo S, Jing W, et al. Primary clofazimine and bedaquiline resistance among isolates from patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2017; 61(6):e00239–17. PMID: 28320727.
Article24. Villellas C, Coeck N, Meehan CJ, Lounis N, de Jong B, Rigouts L, et al. Unexpected high prevalence of resistance-associated Rv0678 variants in MDR-TB patients without documented prior use of clofazimine or bedaquiline. J Antimicrob Chemother. 2017; 72(3):684–690. PMID: 28031270.25. Wen S, Jing W, Zhang T, Zong Z, Xue Y, Shang Y, et al. Comparison of in vitro activity of the nitroimidazoles delamanid and pretomanid against multidrug-resistant and extensively drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2019; 38(7):1293–1296. PMID: 30953211.
Article26. Trébucq A, Schwoebel V, Kashongwe Z, Bakayoko A, Kuaban C, Noeske J, et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis. 2018; 22(1):17–25. PMID: 29149917.
Article27. Lempens P, Decroo T, Aung KJM, Hossain MA, Rigouts L, Meehan CJ, et al. Initial resistance to companion drugs should not be considered an exclusion criterion for the shorter multidrug-resistant tuberculosis treatment regimen. Int J Infect Dis. 2020; 100:357–365. PMID: 32829049.
Article28. Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis patients in Korea 2019. Updated 2020. Accessed March 6, 2021. http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=1&pblctDtaSn=2088.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multidrug-resistant Tuberculosis Spondylitis: A Case Report
- Medical Management of Drug-Resistant Tuberculosis
- Pediatric tuberculosis and drug resistance
- Acquired Drug Resistance during Standardized Treatment with First-line Drugs in Patients with Multidrug-Resistant Tuberculosis
- Drug Resistance Patterns of Multidrug- and Extensively Drug-Resistant Tuberculosis in Korea: Amplification of Resistance to Oral Second-line Drugs