Ann Lab Med.
2017 Jul;37(4):323-326. 10.3343/alm.2017.37.4.323.
Drug Resistance Patterns of Multidrug- and Extensively Drug-Resistant Tuberculosis in Korea: Amplification of Resistance to Oral Second-line Drugs
- Affiliations
-
- 1Department of Laboratory Medicine, Hanyang University Guri Hospital, Guri, Korea.
- 2Korean Institute of Tuberculosis, Cheongju, Korea. leukoso@daum.net
- 3Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2376789
- DOI: http://doi.org/10.3343/alm.2017.37.4.323
Abstract
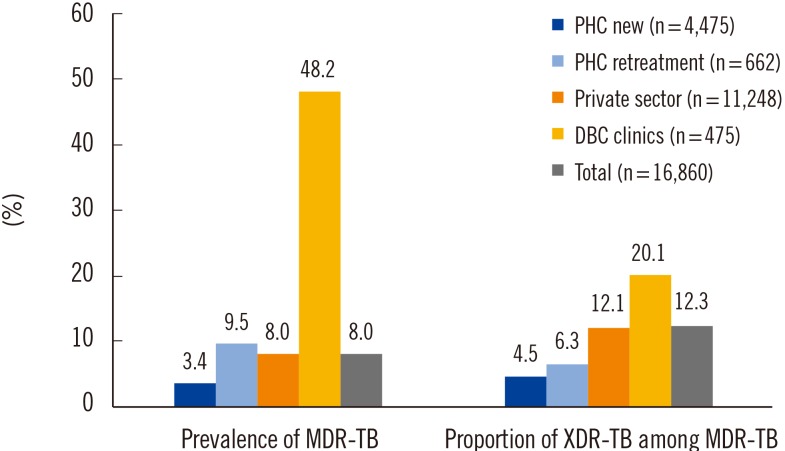
- We aimed to analyze the drug resistance patterns of multidrug-resistant and extensively drug-resistant tuberculosis (TB) and the difference of drug resistance among various settings for health care in Korea. The data of drug susceptibility testing in 2009 was analyzed in order to secure sufficient number of patients from various settings in Korea. Patients were categorized by types of institutions into four groups, which comprised new and previously treated patients from public health care centers (PHC), the private sector, and Double-barred Cross clinics (DBC). The resistance rates to first-line drugs were uniformly high in every group. While the resistance rates to second-line drugs were not as high as first-line drugs, there was a pattern that drug resistance rates were lowest for PHC and highest for DBC. The differences of the resistance rates were more prominent for oral second-line drugs. Our findings implied that drug resistance to oral second-line drugs was significantly amplified during multidrug-resistant-TB treatment in Korea. Therefore, an individualized approach is recommended for treating drug-resistant-TB based on susceptibility testing results to prevent acquisition or amplification of drug resistance.
Keyword
MeSH Terms
Figure
Reference
-
1. WHO. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. http://apps.who.int/iris/bitstream/10665/44286/1/9789241599191_eng.pdf.2. Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008; 178:1075–1082. PMID: 18703792.3. Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007; 11:571–576. PMID: 17439684.4. Park YS, Hong SJ, Boo YK, Hwang ES, Kim HJ, Cho SH, et al. The national status of tuberculosis using nationwide medical records survey of patients with tuberculosis in Korea. Tuberc Respir Dis (Seoul). 2012; 73:48–55. PMID: 23101024.5. Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martín-Casabona N, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007; 13:380–387. PMID: 17552090.6. Kim CK, Joo YT, Lee EP, Park YK, Kim HJ, Kim SJ. Simple, direct drug susceptibility testing technique for diagnosis of drug-resistant tuberculosis in resource-poor settings. Int J Tuberc Lung Dis. 2013; 17:1212–1216. PMID: 23823178.7. Wayne LG. Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am Rev Respir Dis. 1974; 109:147–151. PMID: 4203284.8. Kim JH, Yim JJ. Achievements in and challenges of tuberculosis control in South Korea. Emerg Infect Dis. 2015; 21:1913–1920. PMID: 26485188.9. Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis in Korea, 2015. Cheongju, Korea: Korea Centers for Disease Control and Prevention;2016.10. Zafar Ullah AN, Huque R, Husain A, Akter S, Islam A, Newell JN. Effectiveness of involving the private medical sector in the National TB Control Programme in Bangladesh: evidence from mixed methods. BMJ Open. 2012; 2(6):pii:3001534.11. WHO. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: WHO;2014.12. Yew WW, Lange C. Fluoroquinolone resistance in Mycobacterium tuberculosis. What have we learnt? Int J Tuberc Lung Dis. 2014; 18:1–2. PMID: 24365543.13. Yuen CM, Kurbatova EV, Tupasi T, Caoili JC, Van Der Walt M, Kvasnovsky C, et al. Association between regimen composition and treatment response in patients with multidrug-resistant tuberculosis: A prospective cohort study. PLoS Med. 2015; 12:e1001932. PMID: 26714320.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and treatment of multidrug-resistant tuberculosis
- Medical Treatment of Pulmonary Multidrug-Resistant Tuberculosis
- Medical Management of Drug-Resistant Tuberculosis
- Current status of drug-resistant tuberculosis and its treatment
- Acquired Drug Resistance during Standardized Treatment with First-line Drugs in Patients with Multidrug-Resistant Tuberculosis