Endocrinol Metab.
2021 Jun;36(3):619-627. 10.3803/EnM.2021.974.
Clinicopathological Characteristics and Recurrence-Free Survival of Rare Variants of Papillary Thyroid Carcinomas in Korea: A Retrospective Study
- Affiliations
-
- 1Department of Internal Medicine, Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 3Division of Endocrinology, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 4Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea
- 5Department of Breast and Endocrine Surgery, Hallym University Sacred Heart Hospital, Anyang, Korea
- 6Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- 7Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea
- 8Department of Radiology, School of Medicine, Kangwon National University Hospital, Chuncheon, Korea
- 9Department of Otorhinolaryngology-Head and Neck Surgery, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea
- 10Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
- 11Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2517652
- DOI: http://doi.org/10.3803/EnM.2021.974
Abstract
- Background
We aimed to evaluate the clinicopathological features and biological behaviors of Korean thyroid cancer patients with rare variants of papillary thyroid carcinoma (PTC) to address the ambiguity regarding the prognostic consequences of these variants.
Methods
We retrospectively reviewed the medical records of 5,496 patients who underwent thyroid surgery for PTC, between January and December 2012, in nine tertiary hospitals. Rare PTC variants included tall cell (TCV), columnar cell (CCV), diffuse sclerosing (DSV), cribriform-morular (CMV), solid (SV), hobnail, and Warthin-like variants. Recurrence-free survival (RFS) was defined as the time from the date of thyroidectomy until recurrence.
Results
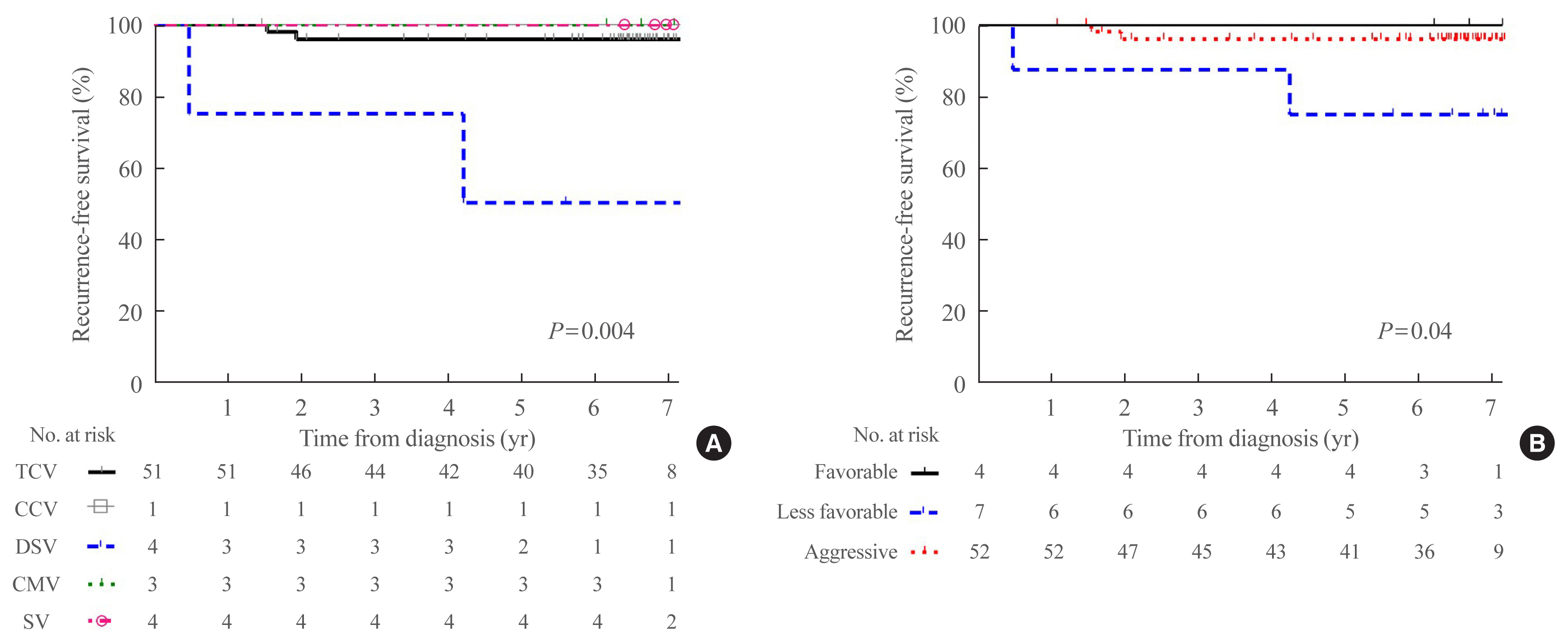
Rare variants accounted for 1.1% (n=63) of the PTC patients; with 0.9% TCV, 0.02% CCV, 0.1% DSV, 0.1% CMV, and 0.1% SV. The mean age of patients and primary tumor size were 42.1±13.1 years and 1.3±0.9 cm, respectively. Extrathyroidal extension and cervical lymph node metastasis were observed in 38 (60.3%) and 37 (58.7%) patients, respectively. Ultrasonographic findings revealed typical malignant features in most cases. During a median follow-up of 7 years, 6.3% of patients experienced a locoregional recurrence. The 5-year RFS rates were 71.4% in patients with DSV or SV, 95.9% for TCV, or CCV, and 100% for other variants. DSV emerged an independent risk factor associated with shorter RFS.
Conclusion
In this multicenter Korean cohort, rare variants accounted for 1.1% of all PTC cases, with TCV being the most frequent subtype. DSV emerged as a significant prognostic factor for RFS.
Keyword
Figure
Reference
-
1. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer. 1998; 83:2638–48.
Article2. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006; 16:1229–42.
Article3. Nikiforov YE, Biddinger PW, Thompson LDR. Diagnostic pathology and molecular genetics of the thyroid. 2nd ed. Philadelphia: Wolters Kluwer;2013.4. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–9.
Article5. Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, et al. Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab. 2016; 101:264–74.
Article6. Bychkov A, Hirokawa M, Jung CK, Liu Z, Zhu Y, Hong SW, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid. 2017; 27:983–4.
Article7. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016; 375:1054–67.
Article8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article9. Kakudo K, Bychkov A, Bai Y, Li Y, Liu Z, Jung CK. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol Int. 2018; 68:641–64.
Article10. Song E, Jeon MJ, Oh HS, Han M, Lee YM, Kim TY, et al. Do aggressive variants of papillary thyroid carcinoma have worse clinical outcome than classic papillary thyroid carcinoma? Eur J Endocrinol. 2018; 179:135–42.
Article11. Ito Y, Hirokawa M, Uruno T, Kihara M, Higashiyama T, Takamura Y, et al. Prevalence and biological behaviour of variants of papillary thyroid carcinoma: experience at a single institute. Pathology. 2008; 40:617–22.
Article12. Jiang C, Cheng T, Zheng X, Hong S, Liu S, Liu J, et al. Clinical behaviors of rare variants of papillary thyroid carcinoma are associated with survival: a population-level analysis. Cancer Manag Res. 2018; 10:465–72.
Article13. Chow SM, Chan JK, Law SC, Tang DL, Ho CM, Cheung WY, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: clinical features and outcome. Eur J Surg Oncol. 2003; 29:446–9.14. Akaishi J, Sugino K, Kameyama K, Masaki C, Matsuzu K, Suzuki A, et al. Clinicopathologic features and outcomes in patients with diffuse sclerosing variant of papillary thyroid carcinoma. World J Surg. 2015; 39:1728–35.
Article15. Chereau N, Giudicelli X, Pattou F, Lifante JC, Triponez F, Mirallie E, et al. Diffuse sclerosing variant of papillary thyroid carcinoma is associated with aggressive histopathological features and a poor outcome: results of a large multicentric study. J Clin Endocrinol Metab. 2016; 101:4603–10.
Article16. Sywak M, Pasieka JL, Ogilvie T. A review of thyroid cancer with intermediate differentiation. J Surg Oncol. 2004; 86:44–54.
Article17. Lee YS, Kim Y, Jeon S, Bae JS, Jung SL, Jung CK. Cytologic, clinicopathologic, and molecular features of papillary thyroid carcinoma with prominent hobnail features: 10 case reports and systematic literature review. Int J Clin Exp Pathol. 2015; 8:7988–97.18. Ieni A, Barresi V, Cardia R, Licata L, Di Bari F, Benvenga S, et al. The micropapillary/hobnail variant of papillary thyroid carcinoma: a review of series described in the literature compared to a series from one southern Italy pathology institution. Rev Endocr Metab Disord. 2016; 17:521–7.
Article19. Teng L, Deng W, Lu J, Zhang J, Ren X, Duan H, et al. Hobnail variant of papillary thyroid carcinoma: molecular profiling and comparison to classical papillary thyroid carcinoma, poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma. Oncotarget. 2017; 8:22023–33.
Article20. Ito M, Bogdanova T, Zurnadzhy L, Saenko V, Rogounovitch T, Mitsutake N, et al. Morphological difference in adult thyroid papillary carcinoma between Japan and Ukraine. Endocr J. 2014; 61:1221–8.
Article21. Sugitani I, Toda K, Yamamoto N, Sakamoto A, Fujimoto Y. Re-evaluation of histopathological factors affecting prognosis of differentiated thyroid carcinoma in an iodine-sufficient country. World J Surg. 2010; 34:1265–73.
Article22. Fukushima M, Ito Y, Hirokawa M, Akasu H, Shimizu K, Miyauchi A. Clinicopathologic characteristics and prognosis of diffuse sclerosing variant of papillary thyroid carcinoma in Japan: an 18-year experience at a single institution. World J Surg. 2009; 33:958–62.
Article23. Falvo L, Giacomelli L, D’Andrea V, Marzullo A, Guerriero G, de Antoni E. Prognostic importance of sclerosing variant in papillary thyroid carcinoma. Am Surg. 2006; 72:438–44.
Article24. Lam AK, Lo CY. Diffuse sclerosing variant of papillary carcinoma of the thyroid: a 35-year comparative study at a single institution. Ann Surg Oncol. 2006; 13:176–81.
Article25. Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito Y, et al. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci. 2008; 99:1908–15.
Article26. Nikiforov YE, Erickson LA, Nikiforova MN, Caudill CM, Lloyd RV. Solid variant of papillary thyroid carcinoma: incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior. Am J Surg Pathol. 2001; 25:1478–84.27. DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and Genetics of Tumours of Endocrine Organs. 3rd ed. Lyon: IARC Press;2004.28. Ito Y, Hirokawa M, Miyauchi A, Higashiyama T, Kihara M, Miya A. Prognostic significance of the proportion of tall cell components in papillary thyroid carcinoma. World J Surg. 2017; 41:742–7.
Article29. Wang X, Cheng W, Liu C, Li J. Tall cell variant of papillary thyroid carcinoma: current evidence on clinicopathologic features and molecular biology. Oncotarget. 2016; 7:40792–9.
Article30. Lee SH, Jung CK, Bae JS, Jung SL, Choi YJ, Kang CS. Liquid-based cytology improves preoperative diagnostic accuracy of the tall cell variant of papillary thyroid carcinoma. Diagn Cytopathol. 2014; 42:11–7.
Article31. Kim SY, Kim T, Kim K, Bae JS, Kim JS, Jung CK. Highly prevalent BRAF V600E and low-frequency TERT promoter mutations underlie papillary thyroid carcinoma in Koreans. J Pathol Transl Med. 2020; 54:310–7.
Article32. Shin JH. Ultrasonographic imaging of papillary thyroid carcinoma variants. Ultrasonography. 2017; 36:103–10.
Article33. Baek HJ, Kim DW, Shin GW, Heo YJ, Baek JW, Lee YJ, et al. Ultrasonographic features of papillary thyroid carcinomas according to their subtypes. Front Endocrinol (Lausanne). 2018; 9:223.
Article34. Choi YJ, Shin JH, Kim JH, Jung SL, Son EJ, Oh YL. Tall cell variant of papillary thyroid carcinoma: sonographic and clinical findings. J Ultrasound Med. 2011; 30:853–8.35. Ha EJ, Moon WJ, Na DG, Lee YH, Choi N, Kim SJ, et al. A multicenter prospective validation study for the Korean Thyroid Imaging Reporting and Data System in patients with thyroid nodules. Korean J Radiol. 2016; 17:811–21.
Article36. Yun MB, Sundar PS, Lan PY, Ying SX, Hua Z. Ultrasonographic features of diffuse sclerosing variant of papillary thyroid carcinoma. J Med Ultrasound. 2011; 19:41–6.
Article37. Chong Y, Shin JH, Oh YL, Han BK, Ko EY. Cribriform-morular variant of papillary thyroid carcinoma: ultrasonographic and clinical characteristics. Thyroid. 2013; 23:45–9.
Article38. Asioli S, Erickson LA, Sebo TJ, Zhang J, Jin L, Thompson GB, et al. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol. 2010; 34:44–52.
Article39. Vuong HG, Kondo T, Pham TQ, Oishi N, Mochizuki K, Nakazawa T, et al. Prognostic significance of diffuse sclerosing variant papillary thyroid carcinoma: a systematic review and meta-analysis. Eur J Endocrinol. 2017; 176:433–41.
Article40. Chen CC, Chen WC, Peng SL, Huang SM. Diffuse sclerosing variant of thyroid papillary carcinoma: diagnostic challenges occur with Hashimoto’s thyroiditis. J Formos Med Assoc. 2013; 112:358–62.
Article41. Sheu SY, Schwertheim S, Worm K, Grabellus F, Schmid KW. Diffuse sclerosing variant of papillary thyroid carcinoma: lack of BRAF mutation but occurrence of RET/PTC rearrangements. Mod Pathol. 2007; 20:779–87.
Article42. Joung JY, Kim TH, Jeong DJ, Park SM, Cho YY, Jang HW, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: major genetic alterations and prognostic implications. Histopathology. 2016; 69:45–53.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Factors for Locally Invasive Papillary Thyroid Carcinomas
- Clinical Characteristics of Papillary Carcinomas with Other Benign Pathologies
- Ultrasonographic imaging of papillary thyroid carcinoma variants
- Expression of Vascular Endothelial Growth Factor (VEGF) in Papillary Thyroid Carcinoma
- A Case of Concurrent Papillary and Medullary Thyroid Carcinomas Detected as Recurrent Medullary Carcinoma after Initial Surgery for Papillary Carcinoma