Korean J Physiol Pharmacol.
2021 Jul;25(4):341-354. 10.4196/kjpp.2021.25.4.341.
Cardamonin exerts a protective effect against autophagy and apoptosis in the testicles of diabetic male rats through the expression of Nrf2 via p62-mediated Keap-1 degradation
- Affiliations
-
- 1Department of Medical Physiology, College of Medicine, Mansoura University, Mansoura 35511, Egypt
- 2Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Mansoura University, Mansoura 35511, Egypt
- 3Department of Anatomy, Faculty of Medicine, King Khalid University, Abha 61421, Saudi Arabia
- 4Department of Histology and Cell Biology, College of Medicine, Benha University, Benha 13511, Egypt
- 5Department of Anatomy, College of Medicine, King Khalid University, Abha 61421, Saudi Arabia
- 6Genomics and Personalized Medicine Unit, College of Medicine, King Khalid University, Abha 61421, Saudi Arabia
- 7Medical Biochemistry Department, College of Medicine, Mansoura University, Mansoura 35511, Egypt
- 8Department of Medical Physiology, College of Medicine, Al-Baha University, Al-Baha 65525, Saudi Arabia
- KMID: 2516873
- DOI: http://doi.org/10.4196/kjpp.2021.25.4.341
Abstract
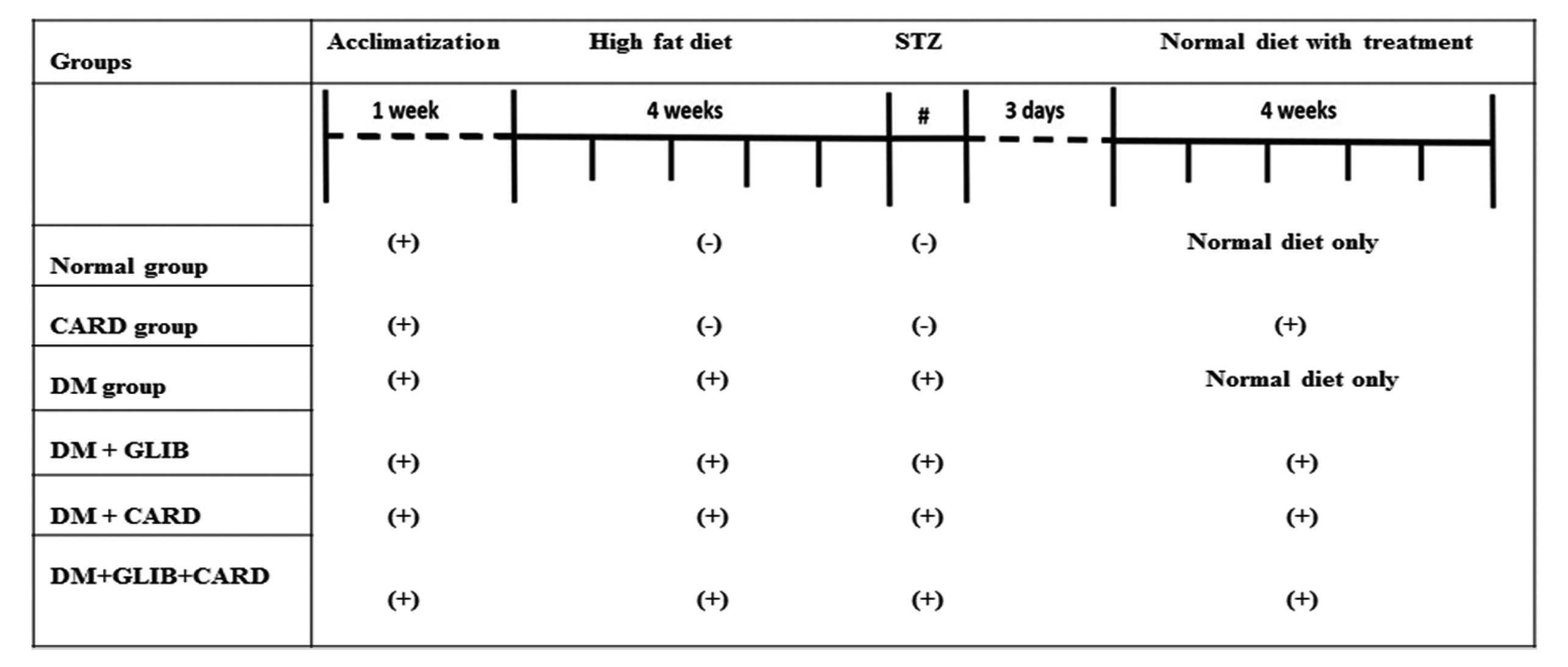
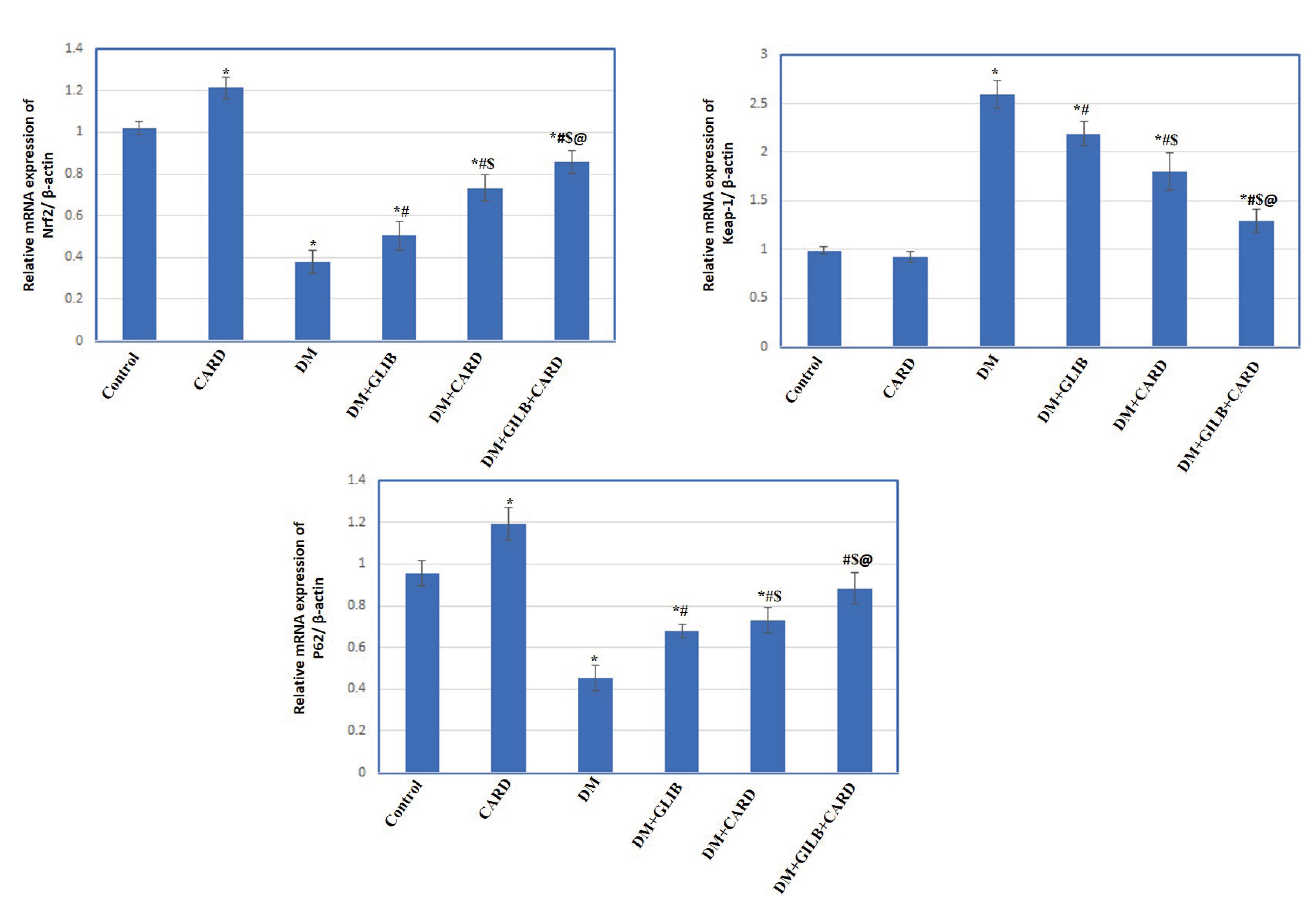
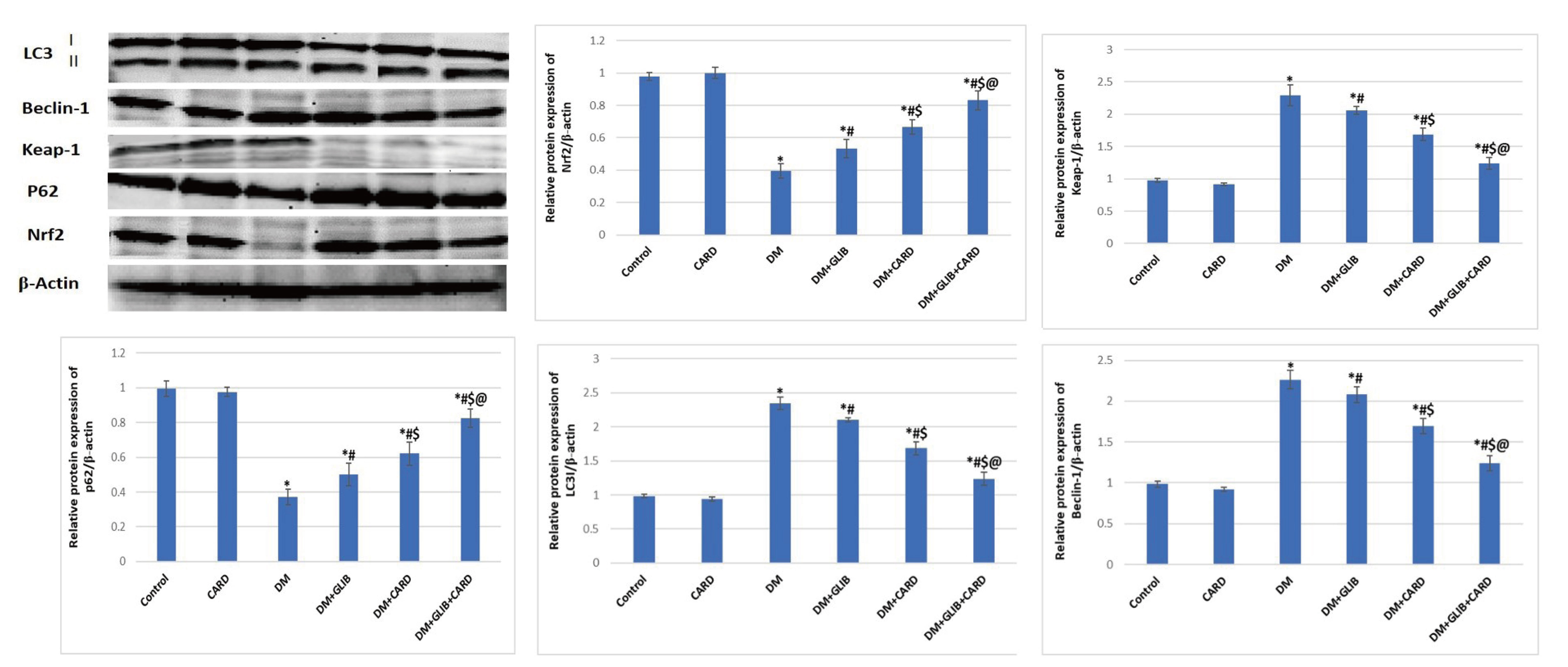
- Cardamonin (CARD) is a chalconoid with anti-inflammatory and antioxidant properties, and it is present in several plants. We sought to explore whether CARD exerts any positive effects against hyperglycemia-induced testicular dysfunction caused by type 2 diabetes and aimed to identify its possible intracellular pathways. Adult male rats were subdivided into six groups: control, CARD, diabetic (DM), DM + glibenclamide (GLIB), DM + CARD and DM + GLIB + CARD. Type 2 DM induced a significant increase in blood glucose and insulin resistance, along with diminished serum insulin, testosterone and gonadotropins levels, which were associated with the impairment of key testicular androgenic enzymes and cellular redox balance. Administration of CARD at a dose of 80 mg/kg for 4 weeks effectively normalized all of these alterations, and the improvement was confirmed by epididymal sperm analysis. After treatment with CARD, the pathological changes in spermatogenic tubules were markedly improved. Significantly, CARD upregulated testicular glucose transporter-8 (GLUT-8) expression and had inhibitory effects on elevated autophagy markers and caspase-3 immunoreactive cells. Furthermore, our results revealed that CARD was able to attenuate damage via activation of Nrf2 through the p62-dependent degradation of testicular anti-Kelch-like ECH-associated protein-1 (Keap-1). In conclusion, this study suggests that CARD provides protection against diabetic stress-mediated testicular damage. The use of CARD with conventional anti-diabetic therapy was associated with improved efficacy compared with conventional therapy alone.
Figure
Reference
-
1. Olokoba AB, Obateru OA, Olokoba LB. 2012; Type 2 diabetes mellitus: a review of current trends. Oman Med J. 27:269–273. DOI: 10.5001/omj.2012.68. PMID: 23071876. PMCID: PMC3464757.
Article2. Ciresi A, Amato MC, Criscimanna A, Mattina A, Vetro C, Galluzzo A, D'Acquisto G, Giordano C. 2007; Metabolic parameters and adipokine profile during GH replacement therapy in children with GH deficiency. Eur J Endocrinol. 156:353–360. DOI: 10.1530/eje.1.02343. PMID: 17322495.
Article3. Ding GL, Liu Y, Liu ME, Pan JX, Guo MX, Sheng JZ, Huang HF. 2015; The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl. 17:948–953. DOI: 10.4103/1008-682X.150844. PMID: 25814158. PMCID: PMC4814953.
Article4. Maresch CC, Stute DC, Alves MG, Oliveira PF, de Kretser DM, Linn T. 2018; Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Hum Reprod Update. 24:86–105. DOI: 10.1093/humupd/dmx033. PMID: 29136166.
Article5. Kautzky-Willer A, Harreiter J, Pacini G. 2016; Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 37:278–316. DOI: 10.1210/er.2015-1137. PMID: 27159875. PMCID: PMC4890267.
Article6. Han XX, Jiang YP, Liu N, Wu J, Yang JM, Li YX, Sun M, Sun T, Zheng P, Jian-Qiang Yu. 2019; Protective effects of Astragalin on spermatogenesis in streptozotocin-induced diabetes in male mice by improving antioxidant activity and inhibiting inflammation. Biomed Pharmacother. 110:561–570. DOI: 10.1016/j.biopha.2018.12.012. PMID: 30537673.
Article7. Sarkar R, Ghosh P, Tripathy A, Ghosh D. 2019; Correction of diabetes-induced testicular dysfunction by a hydro-methanol (60:40) extract of Curcuma amada rhizomes: a dose-dependent study. J Food Biochem. 43:e12829. DOI: 10.1111/jfbc.12829. PMID: 31353516.8. Demirtas L, Guclu A, Erdur FM, Akbas EM, Ozcicek A, Onk D, Turkmen K. 2016; Apoptosis, autophagy & endoplasmic reticulum stress in diabetes mellitus. Indian J Med Res. 144:515–524. DOI: 10.4103/0971-5916.200887. PMCID: PMC5345297.9. Las G, Shirihai OS. 2010; The role of autophagy in β-cell lipotoxicity and type 2 diabetes. Diabetes Obes Metab. 12 Suppl 2:15–19. DOI: 10.1111/j.1463-1326.2010.01268.x. PMID: 21029295. PMCID: PMC3786363.
Article10. Rivera JF, Costes S, Gurlo T, Glabe CG, Butler PC. 2014; Autophagy defends pancreatic β cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest. 124:3489–3500. DOI: 10.1172/JCI71981. PMID: 25036708. PMCID: PMC4109537.
Article11. Abe H, Uchida T, Hara A, Mizukami H, Komiya K, Koike M, Shigihara N, Toyofuku Y, Ogihara T, Uchiyama Y, Yagihashi S, Fujitani Y, Watada H. 2013; Exendin-4 improves β-cell function in autophagy-deficient β-cells. Endocrinology. 154:4512–4524. DOI: 10.1210/en.2013-1578. PMID: 24105478.
Article12. Prabhakar PK, Doble M. 2011; Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Ther Adv Endocrinol Metab. 2:103–114. DOI: 10.1177/2042018811411356. PMID: 23148176. PMCID: PMC3474633.
Article13. Lin YM, Zhou Y, Flavin MT, Zhou LM, Nie W, Chen FC. 2002; Chalcones and flavonoids as anti-tuberculosis agents. Bioorg Med Chem. 10:2795–2802. DOI: 10.1016/S0968-0896(02)00094-9. PMID: 12057669.
Article14. Yang EB, Guo YJ, Zhang K, Chen YZ, Mack P. 2001; Inhibition of epidermal growth factor receptor tyrosine kinase by chalcone derivatives. Biochim Biophys Acta. 1550:144–152. DOI: 10.1016/S0167-4838(01)00276-X. PMID: 11755203.
Article15. Tomecková V, Perjési P, Guzy J, Kusnír J, Chovanová Z, Chavková Z, Mareková M. 2004; Comparison of effect of selected synthetic chalcone analogues on mitochondrial outer membrane determined by fluorescence spectroscopy. J Biochem Biophys Methods. 61:135–141. DOI: 10.1016/j.jbbm.2004.04.010. PMID: 15560929.16. Orlikova B, Tasdemir D, Golais F, Dicato M, Diederich M. 2011; Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 6:125–147. DOI: 10.1007/s12263-011-0210-5. PMID: 21484163. PMCID: PMC3092904.
Article17. Tram LH, Giang PM, Son PT. 2007; Biologically active phenolic constituents from Alpinia gagnepainii K. Schum. (Zingiberaceae). Vietnam J Chem. 45:126–130.18. Yamamoto N, Kawabata K, Sawada K, Ueda M, Fukuda I, Kawasaki K, Murakami A, Ashida H. 2011; Cardamonin stimulates glucose uptake through translocation of glucose transporter-4 in L6 myotubes. Phytother Res. 25:1218–1224. DOI: 10.1002/ptr.3416. PMID: 21305634.
Article19. Qi W, Boliang W, Xiaoxi T, Guoqiang F, Jianbo X, Gang W. 2020; Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed Pharmacother. 122:109547. DOI: 10.1016/j.biopha.2019.109547. PMID: 31918264.
Article20. Ohadoma SC, Michael HU. 2011; Effects of co-administration of methanol leaf extract of Catharanthus roseus on the hypoglycemic activity of metformin and glibenclamide in rats. Asian Pac J Trop Med. 4:475–477. DOI: 10.1016/S1995-7645(11)60129-6. PMID: 21771702.
Article21. Al-Megrin WA, El-Khadragy MF, Hussein MH, Mahgoub S, Abdel-Mohsen DM, Taha H, Bakkar AAA, Abdel Moneim AE, Amin HK. 2020; Green coffea arabica extract ameliorates testicular injury in high-fat diet/streptozotocin-induced diabetes in Rats. J Diabetes Res. 2020:6762709. DOI: 10.1155/2020/6762709. PMID: 32626781. PMCID: PMC7306074.
Article22. Zhang M, Lv XY, Li J, Xu ZG, Chen L. 2008; The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008:704045. DOI: 10.1155/2008/704045. PMID: 19132099. PMCID: PMC2613511.
Article23. Zhou D, Yang Q, Tian T, Chang Y, Li Y, Duan LR, Li H, Wang SW. 2020; Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: Involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed Pharmacother. 126:110075. DOI: 10.1016/j.biopha.2020.110075. PMID: 32179202.
Article24. Muniyappa R, Chen H, Muzumdar RH, Einstein FH, Yan X, Yue LQ, Barzilai N, Quon MJ. 2009; Comparison between surrogate indexes of insulin sensitivity/resistance and hyperinsulinemic euglycemic clamp estimates in rats. Am J Physiol Endocrinol Metab. 297:E1023–E1029. DOI: 10.1152/ajpendo.00397.2009. PMID: 19706785. PMCID: PMC2781355.
Article25. Zhu D, Li X, Macrae VE, Simoncini T, Fu X. 2018; Extragonadal effects of follicle-stimulating hormone on osteoporosis and cardiovascular disease in women during menopausal transition. Trends Endocrinol Metab. 29:571–580. DOI: 10.1016/j.tem.2018.06.001. PMID: 29983231.
Article26. Stoléru SG, Ennaji A, Cournot A, Spira A. 1993; LH pulsatile secretion and testosterone blood levels are influenced by sexual arousal in human males. Psychoneuroendocrinology. 18:205–218. DOI: 10.1016/0306-4530(93)90005-6. PMID: 8516424.
Article27. Chen A, Bookstein JJ, Meldrum DR. 1991; Diagnosis of a testosterone-secreting adrenal adenoma by selective venous catheterization. Fertil Steril. 55:1202–1203. DOI: 10.1016/S0015-0282(16)54378-7. PMID: 1828044.
Article28. Biswas NM, Sen Gupta R, Chattopadhyay A, Choudhury GR, Sarkar M. 2001; Effect of atenolol on cadmium-induced testicular toxicity in male rats. Reprod Toxicol. 15:699–704. DOI: 10.1016/S0890-6238(01)00184-8. PMID: 11738523.
Article29. Ohkawa H, Ohishi N, Yagi K. 1979; Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358. DOI: 10.1016/0003-2697(79)90738-3. PMID: 36810.
Article30. Aebi H. 1984; Catalase in vitro. Methods Enzymol. 105:121–126. DOI: 10.1016/S0076-6879(84)05016-3. PMID: 6727660.31. Beutler E, Duron O, Kelly BM. 1963; Improved method for the determination of blood glutathione. J Lab Clin Med. 61:882–888. PMID: 13967893.32. Kakkar P, Das B, Viswanathan PN. 1984; A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 21:130–132. PMID: 6490072.33. Klinefelter GR, Gray LE Jr, Suarez JD. 1991; The method of sperm collection significantly influences sperm motion parameters following ethane dimethanesulphonate administration in the rat. Reprod Toxicol. 5:39–44. DOI: 10.1016/0890-6238(91)90108-R. PMID: 1666965.
Article34. Zemjanis R. Zemjanis R, editor. 1970. Collection and evaluation of semen. Diagnostic and therapeutic techniques in animal reproduction. 3rd ed. Williams and Wilson Co.;Baltimore: p. 139–156.35. Saber S, Khalil RM, Abdo WS, Nassif D, El-Ahwany E. 2019; Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol Appl Pharmacol. 364:120–132. DOI: 10.1016/j.taap.2018.12.020. PMID: 30594690.
Article36. Muller PY, Janovjak H, Miserez AR, Dobbie Z. 2002; Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 32:1372–1374. 13761378–1379. PMID: 12074169.37. Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. DOI: 10.1006/meth.2001.1262. PMID: 11846609.
Article38. Tabecka-Lonczynska A, Mytych J, Solek P, Koziorowski M. 2020; Autophagy as a consequence of seasonal functions of testis and epididymis in adult male European bison (Bison bonasus, Linnaeus 1758). Cell Tissue Res. 379:613–624. DOI: 10.1007/s00441-019-03111-w. PMID: 31705214.
Article39. Feng XT, Tang SY, Jiang YX, Zhao W. 2017; Anti-diabetic effects of zhuoduqing formula, a Chinese herbal decoction, on a rat model of type 2 diabetes. Afr J Tradit Complement Altern Med. 14:42–50. DOI: 10.21010/ajtcam.v14i3.5. PMID: 28480415. PMCID: PMC5412237.
Article40. Ozdemır O, Akalın PP, Baspınar N, Hatıpoglu F. 2009; Pathological changes in the acute phase of streptozotocin-induced diabetic rats. Bull Vet Inst Pulawy. 53:783–790.41. Kianifard D, Sadrkhanlou RA, Hasanzadeh S. 2012; The ultrastructural changes of the sertoli and leydig cells following streptozotocin induced diabetes. Iran J Basic Med Sci. 15:623–635. PMID: 23493249. PMCID: PMC3586873.42. Kanter M, Aktas C, Erboga M. 2013; Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol Nutr Food Res. 57:1578–1585. DOI: 10.1002/mnfr.201200170. PMID: 22930655.
Article43. Fernandes GS, Fernandez CD, Campos KE, Damasceno DC, Anselmo-Franci JA, Kempinas WD. 2011; Vitamin C partially attenuates male reproductive deficits in hyperglycemic rats. Reprod Biol Endocrinol. 9:100. DOI: 10.1186/1477-7827-9-100. PMID: 21794102. PMCID: PMC3199757.
Article44. Vomund S, Schäfer A, Parnham MJ, Brüne B, von Knethen A. 2017; Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 18:2772. DOI: 10.3390/ijms18122772. PMID: 29261130. PMCID: PMC5751370.
Article45. Komatsu M, Kageyama S, Ichimura Y. 2012; p62/SQSTM1/A170: physiology and pathology. Pharmacol Res. 66:457–462. DOI: 10.1016/j.phrs.2012.07.004. PMID: 22841931.
Article46. Huang C, Lin MZ, Cheng D, Braet F, Pollock CA, Chen XM. 2016; KCa3.1 mediates dysfunction of tubular autophagy in diabetic kidneys via PI3k/Akt/mTOR signaling pathways. Sci Rep. 6:23884. DOI: 10.1038/srep23884. PMID: 27029904. PMCID: PMC4814925.
Article47. Wei Y, Cao XN, Tang XL, Shen LJ, Lin T, He DW, Wu SD, Wei GH. 2018; Urban fine particulate matter (PM2.5) exposure destroys blood-testis barrier (BTB) integrity through excessive ROS-mediated autophagy. Toxicol Mech Methods. 28:302–319. DOI: 10.1080/15376516.2017.1410743. PMID: 29179619.
Article48. Yi WEI, Xiang-Liang T, Yu Z, Bin L, Lian-Ju S, Chun-Lan L, Tao LIN, Da-Wei HE, Sheng-de WU, Guang-Hui WEI. 2018; DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes Dis. 5:263–274. DOI: 10.1016/j.gendis.2018.06.004. PMID: 30320191. PMCID: PMC6176266.
Article49. Huang W, Cao Z, Zhang J, Ji Q, Li Y. 2019; Aflatoxin B1 promotes autophagy associated with oxidative stress-related PI3K/AKT/mTOR signaling pathway in mice testis. Environ Pollut. 255(Pt 2):113317. DOI: 10.1016/j.envpol.2019.113317. PMID: 31610502.50. Su J, Zhou L, Kong X, Yang X, Xiang X, Zhang Y, Li X, Sun L. 2013; Endoplasmic reticulum is at the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in the pathogenesis of diabetes mellitus. J Diabetes Res. 2013:193461. DOI: 10.1155/2013/193461. PMID: 23762873. PMCID: PMC3673337.
Article51. Okouchi M, Okayama N, Aw TY. 2009; Preservation of cellular glutathione status and mitochondrial membrane potential by N-acetylcysteine and insulin sensitizers prevent carbonyl stress-induced human brain endothelial cell apoptosis. Curr Neurovasc Res. 6:267–278. DOI: 10.2174/156720209789630348. PMID: 19807652. PMCID: PMC3170044.
Article52. Wong VY, Keller PM, Nuttall ME, Kikly K, DeWolf WE Jr, Lee D, Ali SM, Nadeau DP, Grygielko ET, Laping NJ, Brooks DP. 2001; Role of caspases in human renal proximal tubular epithelial cell apoptosis. Eur J Pharmacol. 433:135–140. DOI: 10.1016/S0014-2999(01)01517-5. PMID: 11755144.
Article53. Tang XY, Zhang Q, Dai DZ, Ying HJ, Wang QJ, Dai Y. 2008; Effects of strontium fructose 1,6-diphosphate on expression of apoptosis-related genes and oxidative stress in testes of diabetic rats. Int J Urol. 15:251–256. DOI: 10.1111/j.1442-2042.2007.01980.x. PMID: 18304222.
Article54. Boekelheide K, Fleming SL, Johnson KJ, Patel SR, Schoenfeld HA. 2000; Role of Sertoli cells in injury-associated testicular germ cell apoptosis. Proc Soc Exp Biol Med. 225:105–115. DOI: 10.1046/j.1525-1373.2000.22513.x. PMID: 11044252.
Article55. Cai L, Chen S, Evans T, Deng DX, Mukherjee K, Chakrabarti S. 2000; Apoptotic germ-cell death and testicular damage in experimental diabetes: prevention by endothelin antagonism. Urol Res. 28:342–347. DOI: 10.1007/s002400000134. PMID: 11127715.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum Stress-Mediated p62 Downregulation Inhibits Apoptosis via c-Jun Upregulation
- Characterization of LC3 and p62 on Rat Prostate Lobe in Benign Prostate Hyperplasia Animal Model
- Autophagy localization and cytoprotective role in cisplatin-induced acute kidney injury
- Propofol Attenuates Hypoxia/Reoxygenation-Induced Apoptosis and Autophagy in HK-2 Cells by Inhibiting JNK Activation
- Fluoxetine Simultaneously Induces Both Apoptosis and Autophagy in Human Gastric Adenocarcinoma Cells