J Korean Med Sci.
2021 May;36(21):e158. 10.3346/jkms.2021.36.e158.
Positivity of SARS-CoV-2 Antibodies among Korean Healthy Healthcare Workers 1 and 2 Weeks after Second Dose of Pfizer-BioNTech Vaccination
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 2Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Seegene Medical Foundation, Seoul, Korea
- KMID: 2516506
- DOI: http://doi.org/10.3346/jkms.2021.36.e158
Abstract
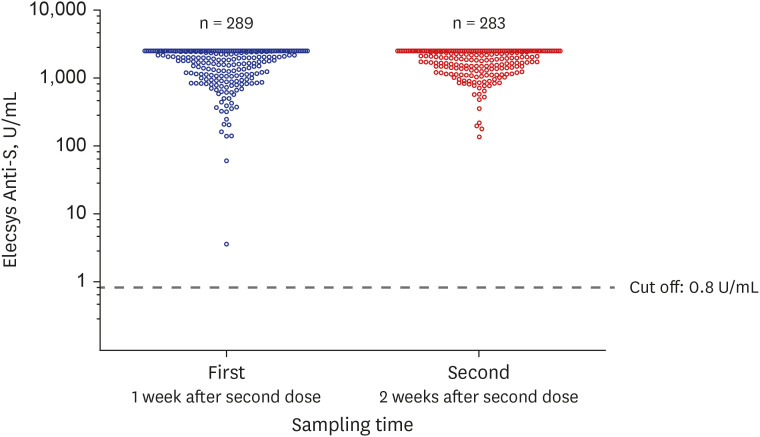
- The antibody titer of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was observed in 289 healthy healthcare workers who had completed the second dose of the Pfizer-BioNTech coronavirus disease 2019 (COVID-19) vaccine. Antibody tests were performed using both the automated electrochemiluminescence immunoassay (ECLIA) and the chromatographic lateral flow immunoassay (LFIA). All subjects had antibodies against the receptor binding domain of the spike protein of SARS-CoV-2 only one week after completing the vaccination, and the antibody titer became significantly higher after another week (P < 0.001). Since there was a large amount of antibody formation within two weeks after completion of vaccination, the less sensitive method, LFIA, also showed high sensitivity. There was no significant difference between whole blood and serum in detecting SARS-CoV-2 antibodies after vaccination. This is an early study of vaccinations among Koreans and is expected to contribute to the establishment of national guidelines on COVID-19 vaccination.
Keyword
Figure
Cited by 2 articles
-
Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine
Yu Min Kang, Dohsik Minn, Jaegyun Lim, Ki-Deok Lee, Dong Ho Jo, Kang-Won Choe, Moon Jung Kim, Jong Min Kim, Kwang Nam Kim
J Korean Med Sci. 2021;36(46):e311. doi: 10.3346/jkms.2021.36.e311.Evaluation of COVID-19 Biokit IgG/IgM Clinical Effectiveness in COVID-19 Vaccinated Individuals
Min Ji Kim, HwaYeon Sun, Byung Wook Yoo
Korean J Health Promot. 2022;22(2):62-67. doi: 10.15384/kjhp.2022.22.2.62.
Reference
-
1. World Health Organization. The coronavirus (COVID-19) dashboard. Updated 2021. Accessed May 2, 2021. https://covid19.who.int/.2. World Health Organization. The COVID-19 candidate vaccine landscape and tracker. Updated 2021. Accessed May 2, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.3. Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020; 586(7830):516–527. PMID: 32967006.
Article4. Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020; 5(1):237. PMID: 33051445.
Article5. Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021; 21(2):73–82. PMID: 33340022.
Article6. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020; 581(7807):215–220. PMID: 32225176.
Article7. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579(7798):270–273. PMID: 32015507.8. Ministry of Food and Drug Safety. Drug information website. Updated 2021. Accessed May 2, 2021. https://nedrug.mfds.go.kr/index.9. Korea Centers for Disease Control and Prevention Agency. COVID-19 vaccine dashboard. Updated 2021. Accessed May 2, 2021. https://ncv.kdca.go.kr/.10. Elecsys®. Anti-SARS-CoV-2 S. Insert (material number 09289267190 and 09289275190). Updated 2020. Accessed May 2, 2021. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2-s.html.11. Elecsys®. Anti-SARS-CoV-2. Insert (material numbers 09203095190 and 09203079190). Updated 2020. Accessed May 2, 2021. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html.12. Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 2015; 72:4–15. PMID: 25908411.
Article13. Engvall E. The ELISA, enzyme-linked immunosorbent assay. Clin Chem. 2010; 56(2):319–320. PMID: 19850633.
Article14. Nicol T, Lefeuvre C, Serri O, Pivert A, Joubaud F, Dubée V, et al. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J Clin Virol. 2020; 129:104511. PMID: 32593133.
Article15. Lassaunière R, Frische A, Harboe ZB, Nielsen ACY, Fomsgaard A, Krogfelt KA, et al. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv. Forthcoming. 2020; DOI: 10.1101/2020.04.09.20056325.
Article16. Serrano MM, Rodríguez DN, Palop NT, Arenas RO, Córdoba MM, Mochón MD, et al. Comparison of commercial lateral flow immunoassays and ELISA for SARS-CoV-2 antibody detection. J Clin Virol. 2020; 129:104529. PMID: 32659710.
Article17. Shincy MR, Govindan V, Sudhakar HH, Venkatesha VT, Padmapriya K, Ravikumar KL. Comparison of performance characteristics between lateral flow, ELISA and electrochemiluminescence immunoassays for the detection of SARS-CoV-2 antibodies among healthcare workers. medRxiv. Forthcoming. 2021; DOI: 10.1101/2021.04.29.21256260.
Article18. Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J Clin Virol. 2020; 129:104480. PMID: 32505777.
Article19. Barnes CO, Jette CA, Abernathy ME, Dam KA, Esswein SR, Gristick HB, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020; 588(7839):682–687. PMID: 33045718.
Article20. Zhang LX, Miao SY, Qin ZH, Wu JP, Chen HY, Sun HB, et al. Preliminary analysis of B- and T-cell responses to SARS-CoV-2. Mol Diagn Ther. 2020; 24(5):601–609. PMID: 32710269.
Article21. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020; 586(7830):594–599. PMID: 32998157.22. Criscuolo E, Diotti RA, Strollo M, Rolla S, Ambrosi A, Locatelli M, et al. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J Med Virol. 2021; 93(4):2160–2167. PMID: 33064340.
Article23. Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020; 586(7830):589–593. PMID: 32785213.24. Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021; 384(1):80–82. PMID: 33270381.
Article25. Doria-Rose N, Suthar MS, Makowski M, O'Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. Forthcoming. 2021; DOI: 10.1056/NEJMc2103916.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-SARS-CoV-2 spike antibody response to the third dose of BNT162b2 mRNA COVID-19 vaccine and associated factors in Japanese hemodialysis patients
- Autoimmune Hepatitis Following Vaccination for SARS-CoV-2 in Korea: Coincidence or Autoimmunity?
- Comparison of Adverse Events of the First Dose and the Second Dose after Vaccination of the COVID-19 Pfizer Vaccine
- Antibody detection in healthcare workers after vaccination with two doses of the BNT162b2 or ChAdOx1 vaccine
- Comparison of the rapidity of SARS-CoV-2 immune responses between primary and booster vaccination for COVID-19