Nutr Res Pract.
2021 Jun;15(3):319-328. 10.4162/nrp.2021.15.3.319.
Extract of Curcuma zedoaria R. prevents atherosclerosis in apolipoprotein E-deficient mice
- Affiliations
-
- 1Herbal Medicine Research Division, Korea Institute of Oriental Medicine, Daejeon 34054, Korea
- 2Department of Korean Life Science and Technology, University of Science and Technology, Daejeon 34113, Korea
- 3Department of Physiology, School of Medicine, Chungnam National University, Daejeon 34134, Korea
- 4Department of Pharmacognosy, College of Pharmacy, Chungnam National University, Daejeon 34134, Korea
- 5Department of Life Science and Biotechnology, Soonchunhyang University, Asan 31538, Korea
- KMID: 2516071
- DOI: http://doi.org/10.4162/nrp.2021.15.3.319
Abstract
- BACKGROUND/OBJECTIVES
Curcuma zedoaria R. (Zingiberaceae) has been used to treat headache, fever, and hypertension-related symptoms in Asian countries, including Korea, China, and Japan. We investigated whether dietary intake of a C. zedoaria extract (CzE) affected atherosclerosis in vivo.
MATERIALS/METHODS
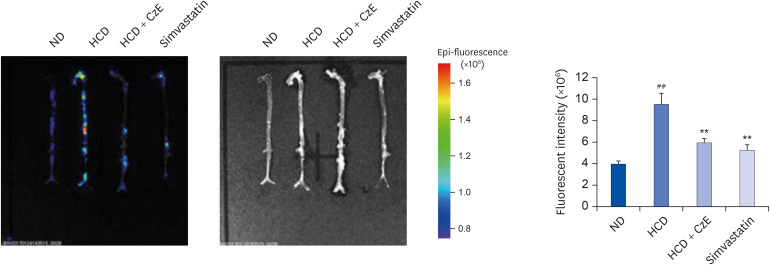
Apolipoprotein E-deficient (ApoEM−/− ) mice (n = 32) were fed a normal diet (ND), a high-cholesterol diet (HCD), an HCD containing CzE (100 mg/kg/day), or an HCD containing simvastatin (10 mg/kg/day) for 12 weeks. The anti-atherosclerotic effects were evaluated by observing changes in fatty streak lesions, immunohistochemical analysis, ex vivo fluorescence imaging, lipid profiles, and western blot analysis.
RESULTS
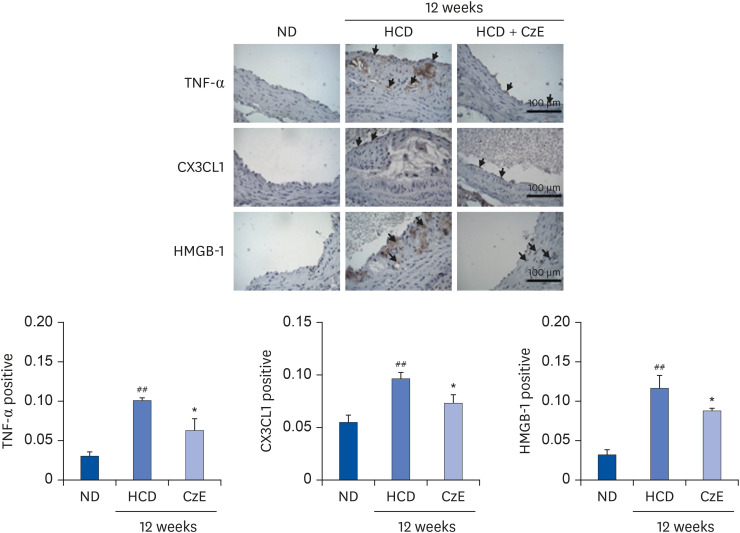
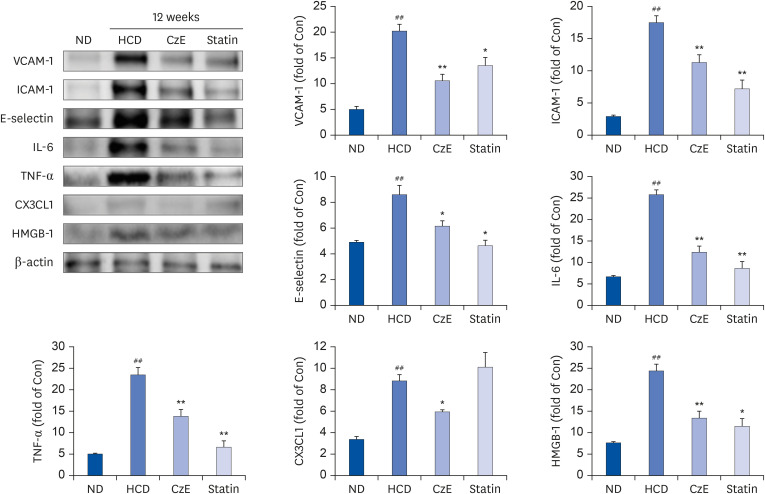
The CzE-fed group showed a 41.6% reduction of atherosclerosis. Furthermore, CzE significantly reduced the levels of serum triglyceride, high-density lipoprotein, the chemokine (C-X3-C-motif ) ligand 1, the adhesion molecules vascular cell adhesion molecule-1, intracellular adhesion molecule-1, and E-selectin; down-regulation of tumor necrosis factor-α, interleukin-6, high mobility group box-1, and cathepsin levels in the aortic sinuses and aortas of ApoE −/− mice were also observed.
CONCLUSIONS
The results suggest that the inclusion of a water extract of C. zedoaria in a HCD is closely correlated with reducing the risk of vascular inflammatory diseases in an ApoE mouse model.
Figure
Reference
-
2. Rai V, Agrawal DK. The role of damage- and pathogen-associated molecular patterns in inflammation-mediated vulnerability of atherosclerotic plaques. Can J Physiol Pharmacol. 2017; 95:1245–1253. PMID: 28746820.
Article3. Barlic J, Zhang Y, Murphy PM. Atherogenic lipids induce adhesion of human coronary artery smooth muscle cells to macrophages by up-regulating chemokine CX3CL1 on smooth muscle cells in a TNFα-NFκB-dependent manner. J Biol Chem. 2007; 282:19167–19176. PMID: 17456471.
Article4. Su Q, Sun Y, Ye Z, Yang H, Kong B, Li L. Pinocembrin protects endothelial cells from oxidized LDL-induced injury. Cytokine. 2018; 111:475–480. PMID: 29914794.
Article5. Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004; 24:1359–1366. PMID: 15178558.
Article6. Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998; 102:576–583. PMID: 9691094.
Article7. Lee TK, Lee D, Lee SR, Ko YJ, Kang KS, Chung SJ, Kim KH. Sesquiterpenes from Curcuma zedoaria rhizomes and their cytotoxicity against human gastric cancer AGS cells. Bioorg Chem. 2019; 87:117–122. PMID: 30884305.8. Hamdi OA, Feroz SR, Shilpi JA, Anouar H, Mukarram AK, Mohamad SB, Tayyab S, Awang K. Spectrofluorometric and molecular docking studies on the binding of curcumenol and curcumenone to human serum albumin. Int J Mol Sci. 2015; 16:5180–5193. PMID: 25756376.9. Akter J, Hossain MA, Takara K, Islam MZ, Hou DX. Antioxidant activity of different species and varieties of turmeric (Curcuma spp): isolation of active compounds. Comp Biochem Physiol C Toxicol Pharmacol. 2019; 215:9–17. PMID: 30266519.10. Ji S, Fattahi A, Raffel N, Hoffmann I, Beckmann MW, Dittrich R, Schrauder M. Antioxidant effect of aqueous extract of four plants with therapeutic potential on gynecological diseases; Semen persicae, Leonurus cardiaca, Hedyotis diffusa, and Curcuma zedoaria . Eur J Med Res. 2017; 22:50. PMID: 29178942.
Article11. Yao C, Jiang J, Tu Y, Ye S, Du H, Zhang Y. β-elemene reverses the drug resistance of A549/DDP lung cancer cells by activating intracellular redox system, decreasing mitochondrial membrane potential and P-glycoprotein expression, and inducing apoptosis. Thorac Cancer. 2014; 5:304–312. PMID: 26767017.
Article12. Jung EB, Trinh TA, Lee TK, Yamabe N, Kang KS, Song JH, Choi S, Lee S, Jang TS, Kim KH, Hwang GS. Curcuzedoalide contributes to the cytotoxicity of Curcuma zedoaria rhizomes against human gastric cancer AGS cells through induction of apoptosis. J Ethnopharmacol. 2018; 213:48–55. PMID: 29102767.13. Chen CC, Chen Y, Hsi YT, Chang CS, Huang LF, Ho CT, Way TD, Kao JY. Chemical constituents and anticancer activity of Curcuma zedoaria roscoe essential oil against non-small cell lung carcinoma cells in vitro and in vivo . J Agric Food Chem. 2013; 61:11418–11427. PMID: 24199734.14. Lee SK, Hong CH, Huh SK, Kim SS, Oh OJ, Min HY, Park KK, Chung WY, Hwang JK. Suppressive effect of natural sesquiterpenoids on inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) activity in mouse macrophage cells. J Environ Pathol Toxicol Oncol. 2002; 21:141–148. PMID: 12086400.
Article15. Makabe H, Maru N, Kuwabara A, Kamo T, Hirota M. Anti-inflammatory sesquiterpenes from Curcuma zedoaria . Nat Prod Res. 2006; 20:680–685. PMID: 16901812.16. Gupta SK, Banerjee AB, Achari B. Isolation of Ethyl p-methoxycinnamate, the major antifungal principle of Curcumba zedoaria . Lloydia. 1976; 39:218–222. PMID: 785141.17. Kimura Y, Sumiyoshi M, Tamaki T. Effects of the extracts and an active compound curcumenone isolated from Curcuma zedoaria rhizomes on alcohol-induced drunkenness in mice. Fitoterapia. 2013; 84:163–169. PMID: 23160092.18. Navarro DF, de Souza MM, Neto RA, Golin V, Niero R, Yunes RA, Delle Monache F, Cechinel Filho V. Phytochemical analysis and analgesic properties of Curcuma zedoaria grown in Brazil. Phytomedicine. 2002; 9:427–432. PMID: 12222663.19. Le TB, Beaufay C, Nghiem DT, Pham TA, Mingeot-Leclercq MP, Quetin-Leclercq J. Evaluation of the anti-trypanosomal activity of Vietnamese essential oils, with emphasis on Curcuma longa L. and its components. Molecules. 2019; 24:1158.20. Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992; 258:468–471. PMID: 1411543.
Article21. Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987; 68:231–240. PMID: 3426656.
Article22. Im AR, Yang WK, Park YC, Kim SH, Chae S. Hepatoprotective effects of insect extracts in an animal model of nonalcoholic fatty liver disease. Nutrients. 2018; 10:735.
Article23. Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007; 116:1052–1061. PMID: 17724271.
Article24. Lee HJ, Im AR, Kim SM, Kang HS, Lee JD, Chae S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. BMC Complement Altern Med. 2018; 18:39. PMID: 29382339.
Article25. Inoue K, Kawahara K, Biswas KK, Ando K, Mitsudo K, Nobuyoshi M, Maruyama I. HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol. 2007; 16:136–143. PMID: 17502242.
Article26. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993; 362:801–809. PMID: 8479518.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibiotic Components from the Rhizomes of Curcuma zedoaria
- Sesquiterpenes from Curcuma zedoaria (Christm.) Rosc. Rhizomes and Their Alpha-Glucosidase Inhibitory Effects
- Effect of Human Bcl-2 Gene Expression on the Peripheral Atherosclerotic Lesions of Apolipoprotein E-Deficient Mouse
- Purple perilla frutescens extracts containing α-asarone inhibit inflammatory atheroma formation and promote hepatic HDL cholesterol uptake in dyslipidemic apoE-deficient mice
- Cytotoxic Activity from Curcuma zedoaria Through Mitochondrial Activation on Ovarian Cancer Cells