Int J Stem Cells.
2021 May;14(2):229-239. 10.15283/ijsc20136.
Improved Differentiation Ability and Therapeutic Effect of miR-23a-3p Expressing Bone Marrow-Derived Mesenchymal Stem Cells in Mice Model with Acute Lung Injury
- Affiliations
-
- 1Department of Intensive Care Medicine, Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2Department of Respiratory and Critical Care Medicine, Shaanxi Provincial People’s Hospital, Xi’an, China
- KMID: 2515999
- DOI: http://doi.org/10.15283/ijsc20136
Abstract
- Background and Objectives
Implantation of bone marrow-derived mesenchymal stem cells (BMSCs) has been recognized as an effective therapy for attenuating acute lung injury (ALI). This study aims to discover microRNA (miRNA)-mediated improvement of BMSCs-based therapeutic effects.
Methods and Results
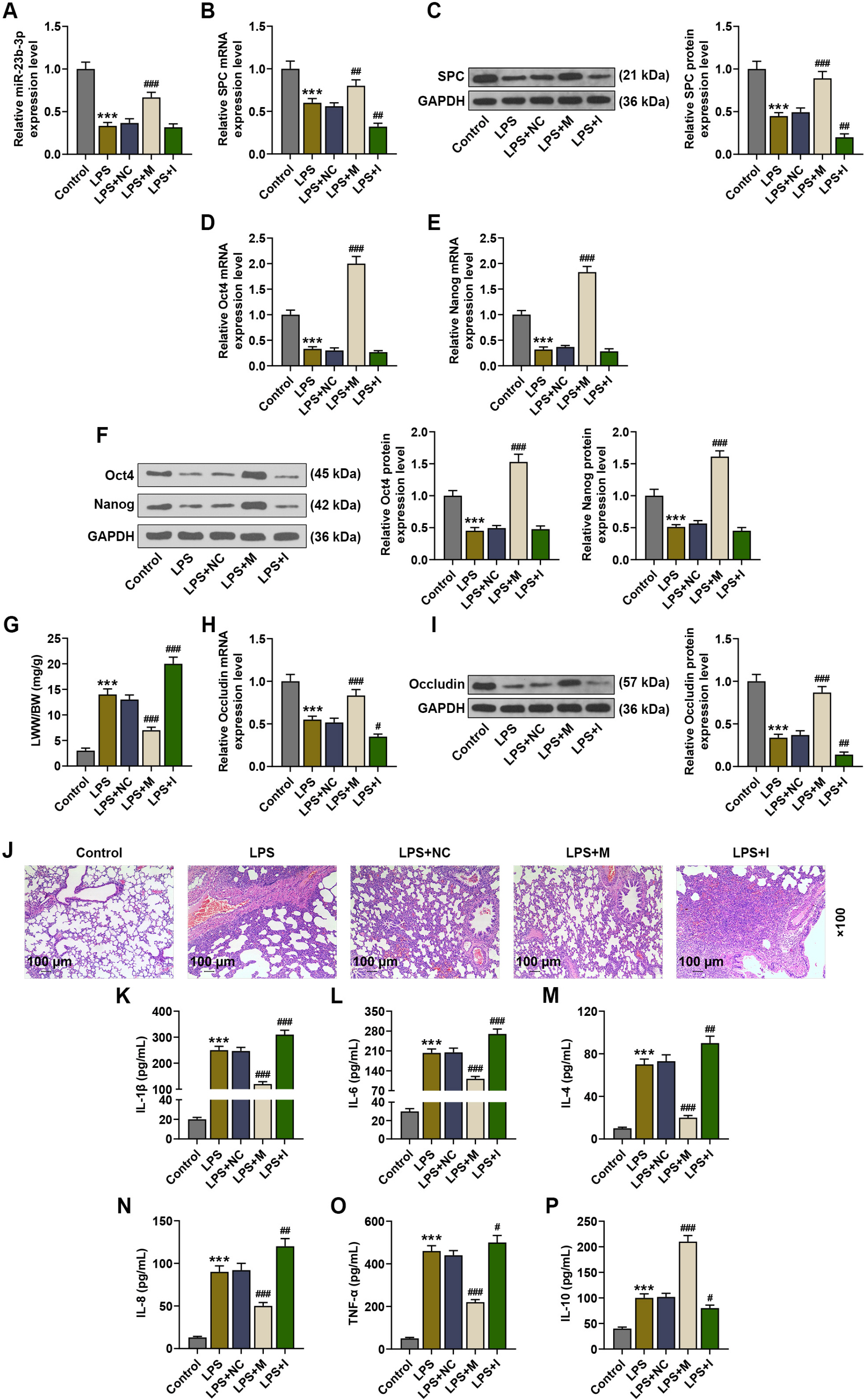
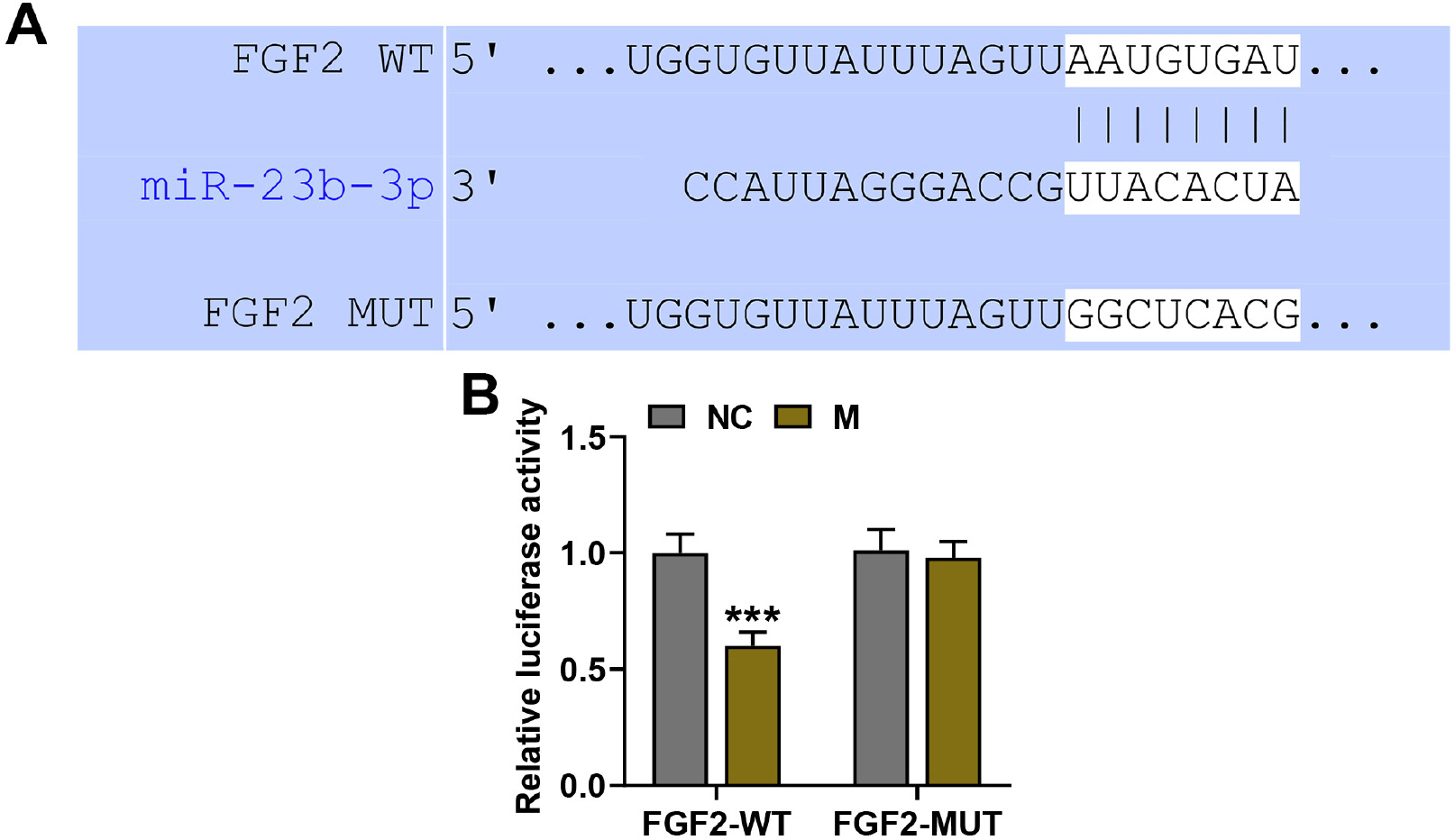
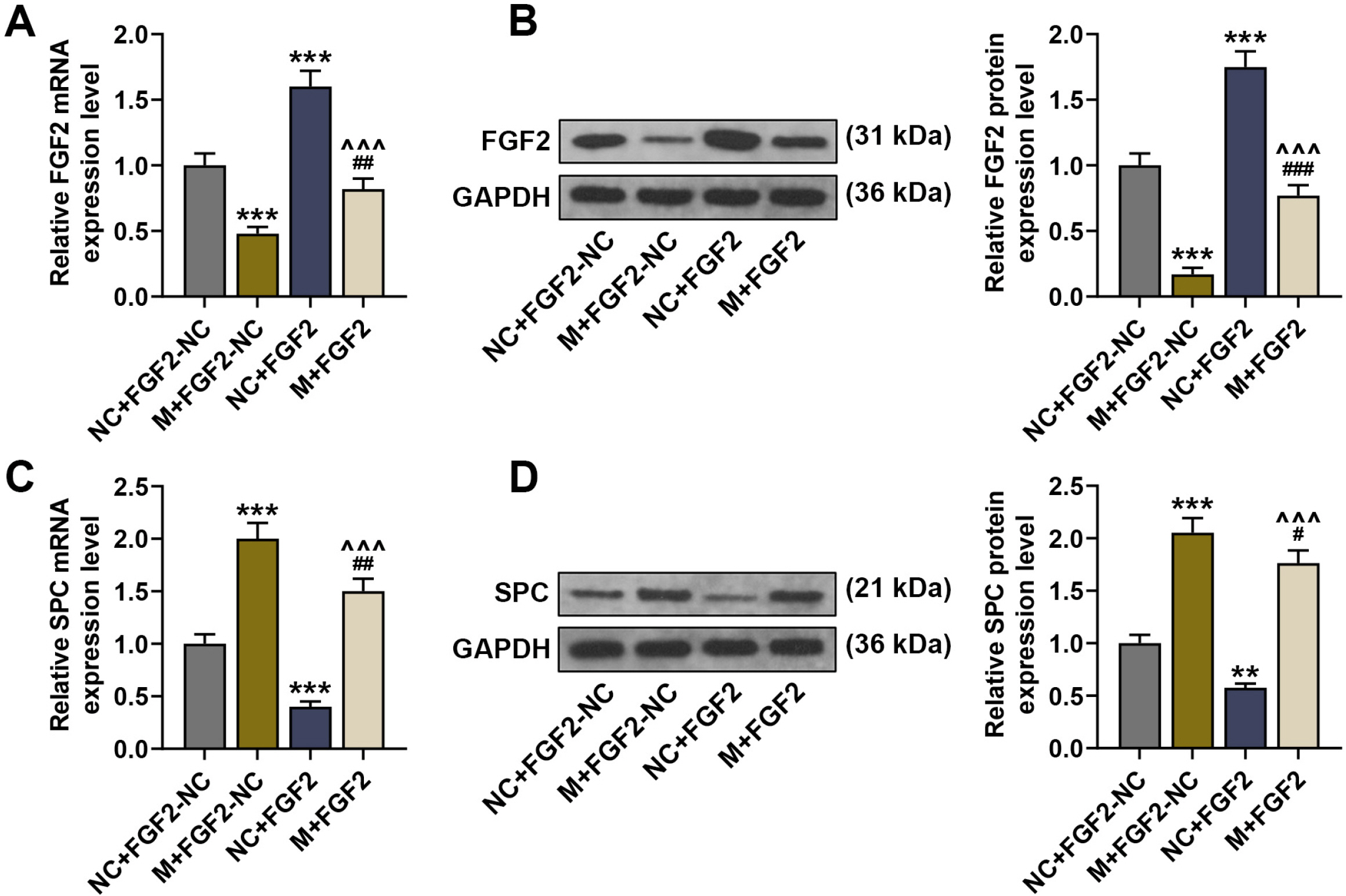
Mice were treated with lipopolysaccharide (LPS) for induction of ALI. BMSCs with lentivirus-mediated expression of miR-23b-3p or fibroblast growth factor 2 (FGF2) were intratracheally injected into the mice with ALI. The expressions of miR-23b-3p, FGF2, Occludin, and surfactant protein C (SPC) in lung tissues were analyzed by immunoblot or quantitative reverse transcription polymerase chain reaction. Histopathological changes in lung tissues were observed via hematoxylin-eosin staining. Lung edema was assessed by the ratio of lung wet weight/body weight (LWW/BW). The levels of interleukin (IL)-1β, IL-6, IL-4, and IL-8 in bronchoalveolar lavage fluid (BALF) were assessed by ELISA. LPS injection downregulated the expressions of miR-23b-3p, SPC and Occludin in the lung tissues, increased the LWW/BW ratio and aggravated histopathological abnormalities, while upregulating IL-1β, IL-6, IL-4, and IL-8 in the BALF. Upregulated miR-23b-3p counteracted LPS-induced effects, whereas downregulated miR-23b-3p intensified LPS-induced effects. FGF2, which was downregulated by miR-23b-3p upregulation, was a target gene of miR-23b-3p. Overexpressing FGF2 downregulated the expressions of miR-23b-3p, SPC and Occludin, increased the LWW/BW ratio and aggravated histopathological abnormalities, while upregulating IL-1β, IL-6, IL-4, and IL-8, and it offset miR-23b-3p upregulation-caused effects on the ALI mice.
Conclusions
Overexpression of miR-23b-3p in BMSCs strengthened BMSC-mediated protection against LPS-induced mouse acute lung injury via targeting FGF2.
Keyword
Figure
Reference
-
References
1. Confalonieri M, Salton F, Fabiano F. 2017; Acute respiratory distress syndrome. Eur Respir Rev. 26:160116. DOI: 10.1183/16000617.0116-2016. PMID: 28446599.
Article2. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A. 2016; Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 315:788–800. DOI: 10.1001/jama.2016.0291. PMID: 26903337.
Article3. Cardenes N, Aranda-Valderrama P, Carney JP, Sellares Torres J, Alvarez D, Kocyildirim E, Wolfram Smith JA, Ting AE, Lagazzi L, Yu Z, Mason S, Santos E, Lopresti BJ, Rojas M. 2019; Cell therapy for ARDS: efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir Res. 6:e000308. DOI: 10.1136/bmjresp-2018-000308. PMID: 30713713. PMCID: PMC6339992.
Article4. Lu H, Cook T, Poirier C, Merfeld-Clauss S, Petrache I, March KL, Bogatcheva NV. 2016; Pulmonary retention of adipose stromal cells following intravenous delivery is markedly altered in the presence of ARDS. Cell Transplant. 25:1635–1643. DOI: 10.3727/096368915X690189. PMID: 26609693.
Article5. Parekkadan B, Milwid JM. 2010; Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 12:87–117. DOI: 10.1146/annurev-bioeng-070909-105309. PMID: 20415588. PMCID: PMC3759519.
Article6. Park J, Kim S, Lim H, Liu A, Hu S, Lee J, Zhuo H, Hao Q, Matthay MA, Lee JW. 2019; Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 74:43–50. DOI: 10.1136/thoraxjnl-2018-211576. PMID: 30076187. PMCID: PMC6295323.
Article7. Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. 2019; Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 7:154–162. DOI: 10.1016/S2213-2600(18)30418-1. PMID: 30455077. PMCID: PMC7597675.
Article8. Bartel DP. 2004; MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116:281–297. DOI: 10.1016/S0092-8674(04)00045-5. PMID: 14744438.9. Gu W, Hong X, Le Bras A, Nowak WN, Issa Bhaloo S, Deng J, Xie Y, Hu Y, Ruan XZ, Xu Q. 2018; Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J Biol Chem. 293:8089–8102. DOI: 10.1074/jbc.RA118.001739. PMID: 29643181. PMCID: PMC5971462.
Article10. Li Y, Ke J, Peng C, Wu F, Song Y. 2018; microRNA-300/NAMPT regulates inflammatory responses through activation of AMPK/mTOR signaling pathway in neonatal sepsis. Biomed Pharmacother. 108:271–279. DOI: 10.1016/j.biopha.2018.08.064. PMID: 30223098.
Article11. Cai ZG, Zhang SM, Zhang Y, Zhou YY, Wu HB, Xu XP. 2012; MicroRNAs are dynamically regulated and play an important role in LPS-induced lung injury. Can J Physiol Pharmacol. 90:37–43. DOI: 10.1139/y11-095. PMID: 22185353.
Article12. Parker MI, Palladino MA. 2017; MicroRNAs downregulated following immune activation of rat testis. Am J Reprod Immunol. 77:e12673. DOI: 10.1111/aji.12673. PMID: 28328045.13. Raut A, Khanna A. 2016; Enhanced expression of hepatocyte-specific microRNAs in valproic acid mediated hepatic trans-differentiation of human umbilical cord derived mesenchymal stem cells. Exp Cell Res. 343:237–247. DOI: 10.1016/j.yexcr.2016.03.015. PMID: 27001466.
Article14. Beenken A, Mohammadi M. 2009; The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 8:235–253. DOI: 10.1038/nrd2792. PMID: 19247306. PMCID: PMC3684054.
Article15. Wullaert A, Bonnet MC, Pasparakis M. 2011; NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 21:146–158. DOI: 10.1038/cr.2010.175. PMID: 21151201. PMCID: PMC3193399.
Article16. Cheng D, Zhu C, Liang Y, Xing Y, Shi C. 2020; MiR-424 overexpression protects alveolar epithelial cells from LPS-induced apoptosis and inflammation by targeting FGF2 via the NF-κB pathway. Life Sci. 242:117213. DOI: 10.1016/j.lfs.2019.117213. PMID: 31881228.
Article17. Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. DOI: 10.1006/meth.2001.1262. PMID: 11846609.
Article18. Li X, Jamal M, Guo P, Jin Z, Zheng F, Song X, Zhan J, Wu H. 2019; Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed Pharmacother. 118:109363. DOI: 10.1016/j.biopha.2019.109363. PMID: 31545277.
Article19. Zhang L, Li Q, Liu W, Liu Z, Shen H, Zhao M. 2019; Mesen-chymal stem cells alleviate acute lung injury and inflammatory responses induced by paraquat poisoning. Med Sci Monit. 25:2623–2632. DOI: 10.12659/MSM.915804. PMID: 30967525. PMCID: PMC6474293.
Article20. Li L, Dong L, Wang Y, Zhang X, Yan J. 2018; Lats1/2-mediated alteration of hippo signaling pathway regulates the fate of bone marrow-derived mesenchymal stem cells. Biomed Res Int. 2018:4387932. DOI: 10.1155/2018/4387932. PMID: 30671453. PMCID: PMC6323436.21. Qin H, Zhao A. 2020; Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell. 11:707–722. DOI: 10.1007/s13238-020-00738-2. PMID: 32519302. PMCID: PMC7282699.
Article22. Jiang J, Song Z, Zhang L. 2018; miR-155-5p promotes progression of acute respiratory distress syndrome by inhibiting differentiation of bone marrow mesenchymal stem cells to alveolar type II epithelial cells. Med Sci Monit. 24:4330–4338. DOI: 10.12659/MSM.910316. PMID: 29936517. PMCID: PMC6049011.
Article23. Rajasekaran S, Pattarayan D, Rajaguru P, Sudhakar Gandhi PS, Thimmulappa RK. 2016; MicroRNA regulation of acute lung injury and acute respiratory distress syndrome. J Cell Physiol. 231:2097–2106. DOI: 10.1002/jcp.25316. PMID: 26790856.
Article24. Cardinal-Fernández P, Ferruelo A, Esteban A, Lorente JA. 2016; Characteristics of microRNAs and their potential relevance for the diagnosis and therapy of the acute respiratory distress syndrome: from bench to bedside. Transl Res. 169:102–111. DOI: 10.1016/j.trsl.2015.11.004. PMID: 26687392.
Article25. Suo T, Chen GZ, Huang Y, Zhao KC, Wang T, Hu K. 2018; miRNA-1246 suppresses acute lung injury-induced inflammation and apoptosis via the NF-κB and Wnt/β-ca-tenin signal pathways. Biomed Pharmacother. 108:783–791. DOI: 10.1016/j.biopha.2018.09.046. PMID: 30253370.
Article26. Zhang W, Lu F, Xie Y, Lin Y, Zhao T, Tao S, Lai Z, Wei N, Yang R, Shao Y, He J. 2019; miR-23b negatively regulates sepsis-induced inflammatory responses by targeting ADAM10 in human THP-1 monocytes. Mediators Inflamm. 2019:5306541. DOI: 10.1155/2019/5306541. PMID: 31780861. PMCID: PMC6875296.
Article27. Lecchi C, Dalla Costa E, Lebelt D, Ferrante V, Canali E, Ceciliani F, Stucke D, Minero M. 2018; Circulating miR-23b-3p, miR-145-5p and miR-200b-3p are potential biomarkers to monitor acute pain associated with laminitis in horses. Animal. 12:366–375. DOI: 10.1017/S1751731117001525. PMID: 28689512.
Article28. Dong L, Li L. 2019; Lats2-underexpressing bone marrow-derived mesenchymal stem cells ameliorate LPS-induced acute lung injury in mice. Mediators Inflamm. 2019:4851431. DOI: 10.1155/2019/4851431. PMID: 31772503. PMCID: PMC6854183.
Article29. Wang L, Shi M, Tong L, Wang J, Ji S, Bi J, Chen C, Jiang J, Bai C, Zhou J, Song Y. 2019; Lung-resident mesenchymal stem cells promote repair of LPS-induced acute lung injury via regulating the balance of regulatory T cells and Th17 cells. Inflammation. 42:199–210. DOI: 10.1007/s10753-018-0884-6. PMID: 30187337.
Article30. Jin H, Ciechanowicz AK, Kaplan AR, Wang L, Zhang PX, Lu YC, Tobin RE, Tobin BA, Cohn L, Zeiss CJ, Lee PJ, Bruscia EM, Krause DS. 2018; Surfactant protein C dampens inflammation by decreasing JAK/STAT activation during lung repair. Am J Physiol Lung Cell Mol Physiol. 314:L882–L892. DOI: 10.1152/ajplung.00418.2017. PMID: 29345196. PMCID: PMC6008135.
Article31. Rao R. 2009; Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci. 1165:62–68. DOI: 10.1111/j.1749-6632.2009.04054.x. PMID: 19538289. PMCID: PMC6202026.
Article32. Zhang YL, Li QQ, Guo W, Huang Y, Yang J. 2007; Effects of chronic ethanol ingestion on tight junction proteins and barrier function of alveolar epithelium in the rat. Shock. 28:245–252. DOI: 10.1097/SHK.0b013e31803404a9. PMID: 17515855.
Article33. Crosby LM, Waters CM. 2010; Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 298:L715–L731. DOI: 10.1152/ajplung.00361.2009. PMID: 20363851. PMCID: PMC2886606.
Article34. Afonso-Grunz F, Müller S. 2015; Principles of miRNA-mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci. 72:3127–3141. DOI: 10.1007/s00018-015-1922-2. PMID: 26037721.
Article35. Shao X, Chen S, Yang D, Cao M, Yao Y, Wu Z, Li N, Shen N, Li X, Song X, Qian Y. 2017; FGF2 cooperates with IL-17 to promote autoimmune inflammation. Sci Rep. 7:7024. DOI: 10.1038/s41598-017-07597-8. PMID: 28765647. PMCID: PMC5539112.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MiR-148a-3p Regulates the Invasion and Odontoblastic Differentiation of Human Dental Pulp Stem Cells via the Wnt1/β-Catenin Pathway
- MiR-29a-3p Inhibits Proliferation and Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells via Targeting FOXO3 and Repressing Wnt/β-Catenin Signaling in Steroid-Associated Osteonecrosis
- Intra-Articular Injection of miR-29a-3p of BMSCs Promotes Cartilage Self-Repairing and Alleviates Pain in the Rat Osteoarthritis
- Effects of Transplantation with Marrow-Derived Mesenchymal Stem Cells Modified with Survivin on Renal Ischemia-Reperfusion Injury in Mice
- MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN