Yonsei Med J.
2014 Jul;55(4):1130-1137. 10.3349/ymj.2014.55.4.1130.
Effects of Transplantation with Marrow-Derived Mesenchymal Stem Cells Modified with Survivin on Renal Ischemia-Reperfusion Injury in Mice
- Affiliations
-
- 1Department of Urology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China. kyl361@yeah.net
- KMID: 2130843
- DOI: http://doi.org/10.3349/ymj.2014.55.4.1130
Abstract
- PURPOSE
To determine whether renal injury induced by ischemia-reperfusion (I/R) could be further improved by mesenchymal stem cells (MSCs) modified with survivin.
MATERIALS AND METHODS
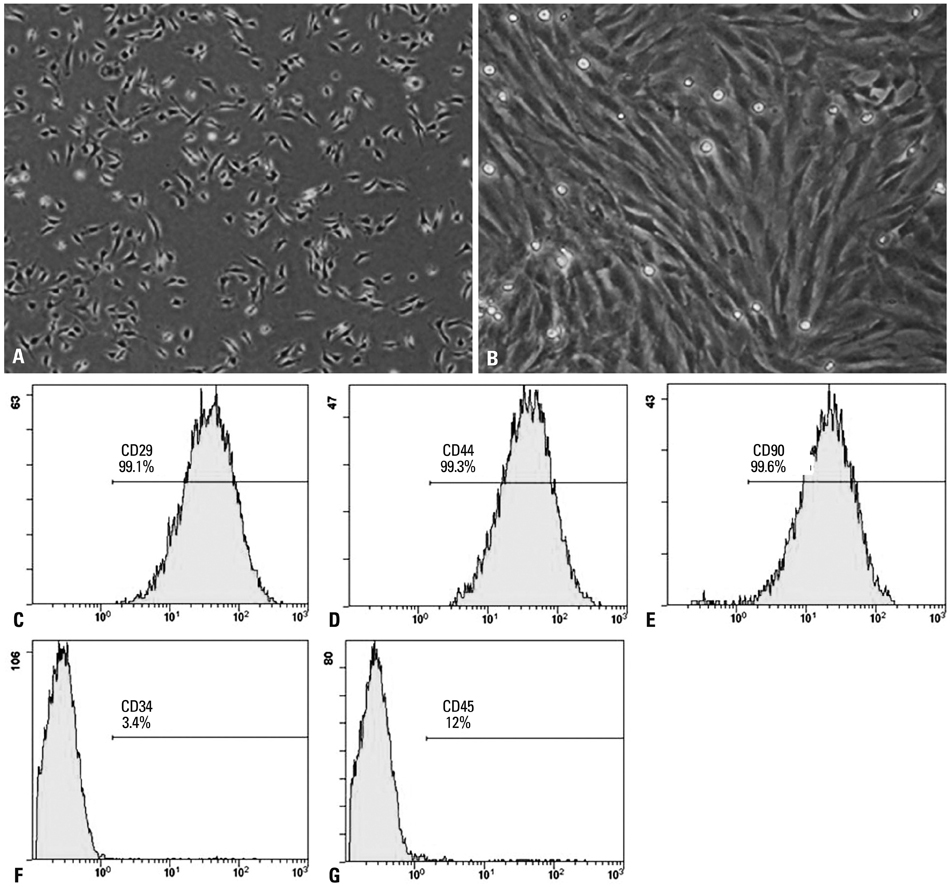
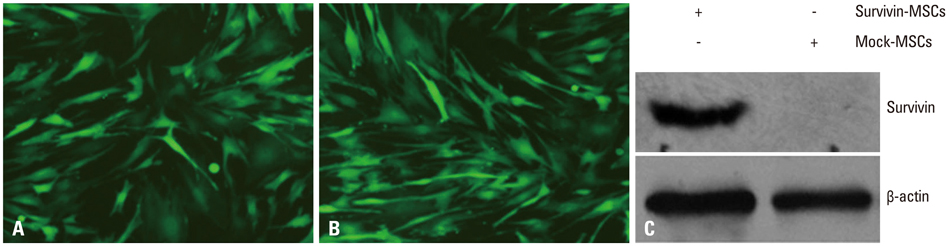
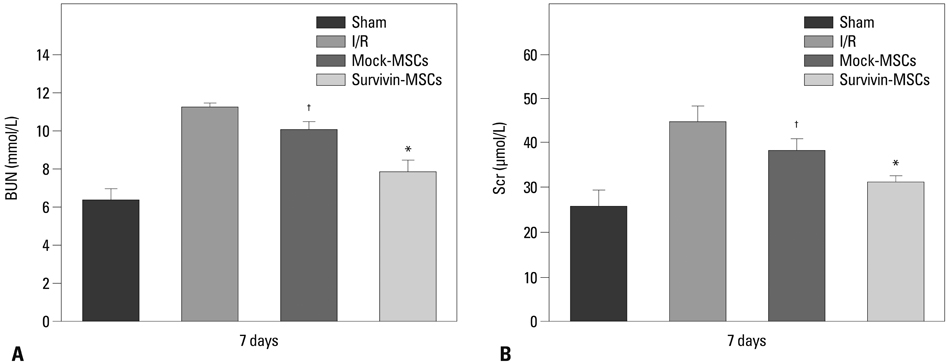
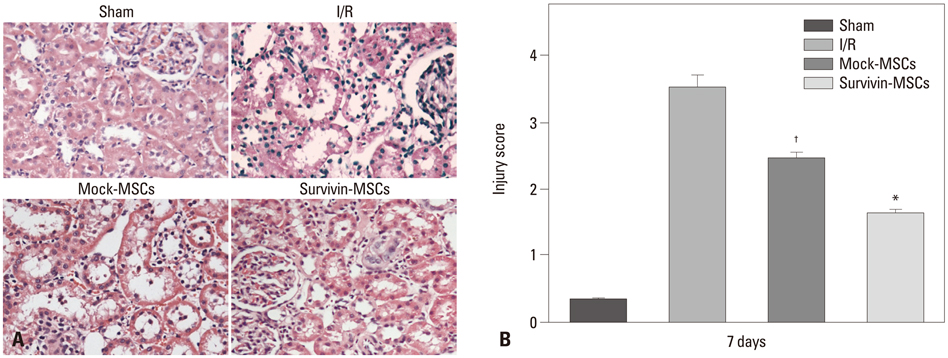
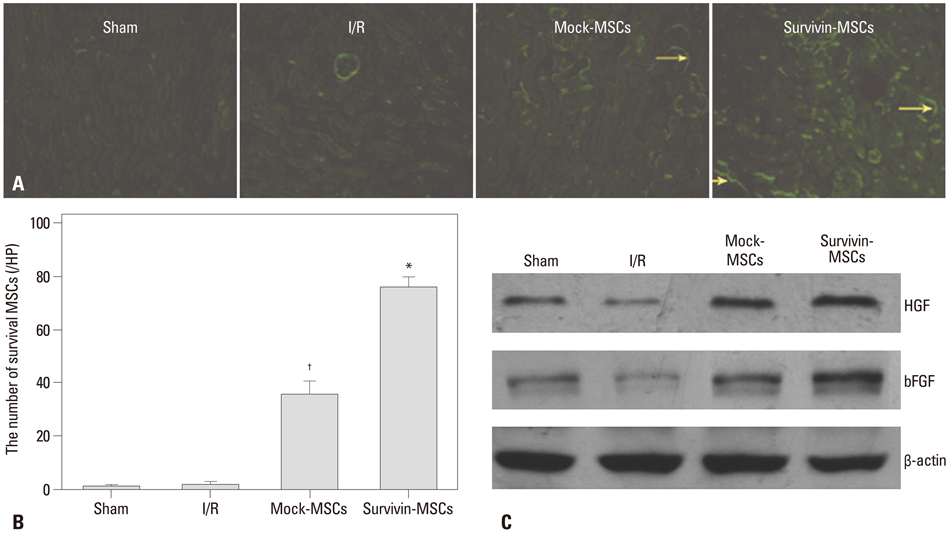
Lentiviral vectors were used to introduce the survivin gene into MSCs and the MSCs modified with survivin were transplanted into established mice models of renal I/R injury. Seven days later, serum creatinine (Scr) and blood urea nitrogen (BUN) were measured and the survival of MSCs was determined. Hematoxylin and eosin staining was used to assess renal pathological change. The expressions of hepatocyte growth factor (HGF) and basic fibroblast growth factor (bFGF) in kidney tissue were detected by western blot.
RESULTS
Mice transplanted with survivin-modified MSCs demonstrated good renal function recovery with Scr and BUN decline close to normal levels and improvement of renal I/R injury repair. Additionally, the survival of transplanted MSCs modified with survivin was enhanced and the expression of HGF and bFGF in kidney tissue was increased.
CONCLUSION
Our results demonstrated that MSCs engineered to over-express survivin could enhance their therapeutic effect on renal I/R injury in mice, probably via the improved survival ability of MSCs and increased production of protective cytokines in ischemic tissue.
MeSH Terms
Figure
Reference
-
1. Snoeijs MG, Vink H, Voesten N, Christiaans MH, Daemen JW, Peppelenbosch AG, et al. Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am J Physiol Renal Physiol. 2010; 299:F1134–F1140.
Article2. Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009; 119:495–502.3. Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011; 7:189–200.
Article4. Chua HR, Glassford N, Bellomo R. Acute kidney injury after cardiac arrest. Resuscitation. 2012; 83:721–727.
Article5. Henry SD, Guarrera JV. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev (Orlando). 2012; 26:163–175.
Article6. Hu L, Yang C, Zhao T, Xu M, Tang Q, Yang B, et al. Erythropoietin ameliorates renal ischemia and reperfusion injury via inhibiting tubulointerstitial inflammation. J Surg Res. 2012; 176:260–266.
Article7. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001; 105:369–377.
Article8. Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004; 287:H2670–H2676.
Article9. Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005; 289:F31–F42.
Article10. Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005; 68:1613–1617.
Article11. Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005; 68:1956–1961.
Article12. Mylotte LA, Duffy AM, Murphy M, O'Brien T, Samali A, Barry F, et al. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. 2008; 26:1325–1336.
Article13. Chen J, Li Y, Wang L, Lu M, Chopp M. Caspase inhibition by Z-VAD increases the survival of grafted bone marrow cells and improves functional outcome after MCAo in rats. J Neurol Sci. 2002; 199:17–24.
Article14. Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, et al. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005; 19:183–193.
Article15. Fan L, Lin C, Zhuo S, Chen L, Liu N, Luo Y, et al. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail. 2009; 11:1023–1030.
Article16. Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng R, et al. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J Transl Med. 2011; 9:105.
Article17. Deng W, Bivalacqua TJ, Chattergoon NN, Hyman AL, Jeter JR Jr, Kadowitz PJ. Adenoviral gene transfer of eNOS: high-level expression in ex vivo expanded marrow stromal cells. Am J Physiol Cell Physiol. 2003; 285:C1322–C1329.
Article18. Villanueva S, Suazo C, Santapau D, Pérez F, Quiroz M, Carreño JE, et al. NFAT5 is activated by hypoxia: role in ischemia and reperfusion in the rat kidney. PLoS One. 2012; 7:e39665.
Article19. Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004; 362:200–203.
Article20. Zhao Y, Li T, Wei X, Bianchi G, Hu J, Sanchez PG, et al. Mesenchymal stem cell transplantation improves regional cardiac remodeling following ovine infarction. Stem Cells Transl Med. 2012; 1:685–695.
Article21. Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003; 9:1195–1201.
Article22. Huls M, Russel FG, Masereeuw R. Insights into the role of bone marrow-derived stem cells in renal repair. Kidney Blood Press Res. 2008; 31:104–110.
Article23. Chiou SK, Jones MK, Tarnawski AS. Survivin - an anti-apoptosis protein: its biological roles and implications for cancer and beyond. Med Sci Monit. 2003; 9:PI25–PI29.24. Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007; 292:F1626–F1635.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alleviation of renal ischemia/reperfusion injury by exosomes from induced pluripotent stem cell-derived mesenchymal stem cells
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Bone marrow-derived mesenchymal stem cells reactive oxygen species-responsively secreting hepatocyte growth factor for effective treatment of ischemia-reperfusion injury in liver transplantation
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- Urine-derived stem cell attenuated renal fibrosis via Klotho activation