Int J Stem Cells.
2021 May;14(2):180-190. 10.15283/ijsc20089v.
Inhibition of MUC1-C Increases ROS and Cell Death in Mouse Embryonic Stem Cells
- Affiliations
-
- 1Department of Biochemistry, College of Natural Sciences, Chungbuk National University, Cheongju, Korea
- 2Biotechnology Research Institute, Chungbuk National University, Cheongju, Korea
- 3Department of Microbiology, Chonbuk National University Medical School, Jeonju, Korea
- KMID: 2515994
- DOI: http://doi.org/10.15283/ijsc20089v
Abstract
- Background and Objectives
Embryonic stem (ES) cells have the capacity to self-renew and generate all types of cells. MUC1-C, a cytoplasmic subunit of MUC1, is overexpressed in various carcinomas and mediates signaling pathways to regulate intracellular metabolic processes and gene expression involved in the maintenance of cancer cells. However, the functional role of MUC1-C in ES cells is not well understood. In this study, we investigated the role of MUC1-C on growth, survival,: and differentiation of mouse ES (mES) cells.
Methods and Results
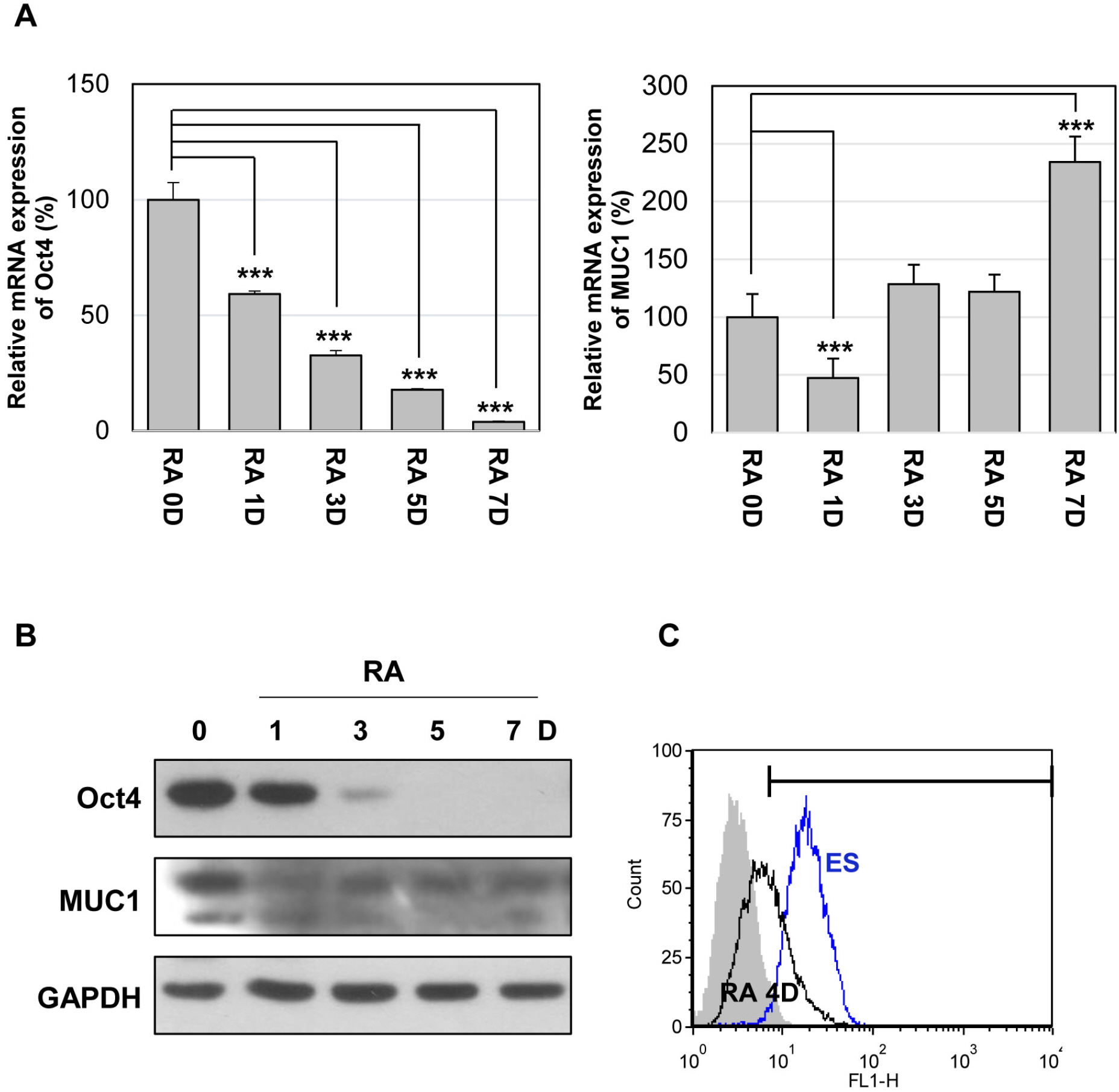
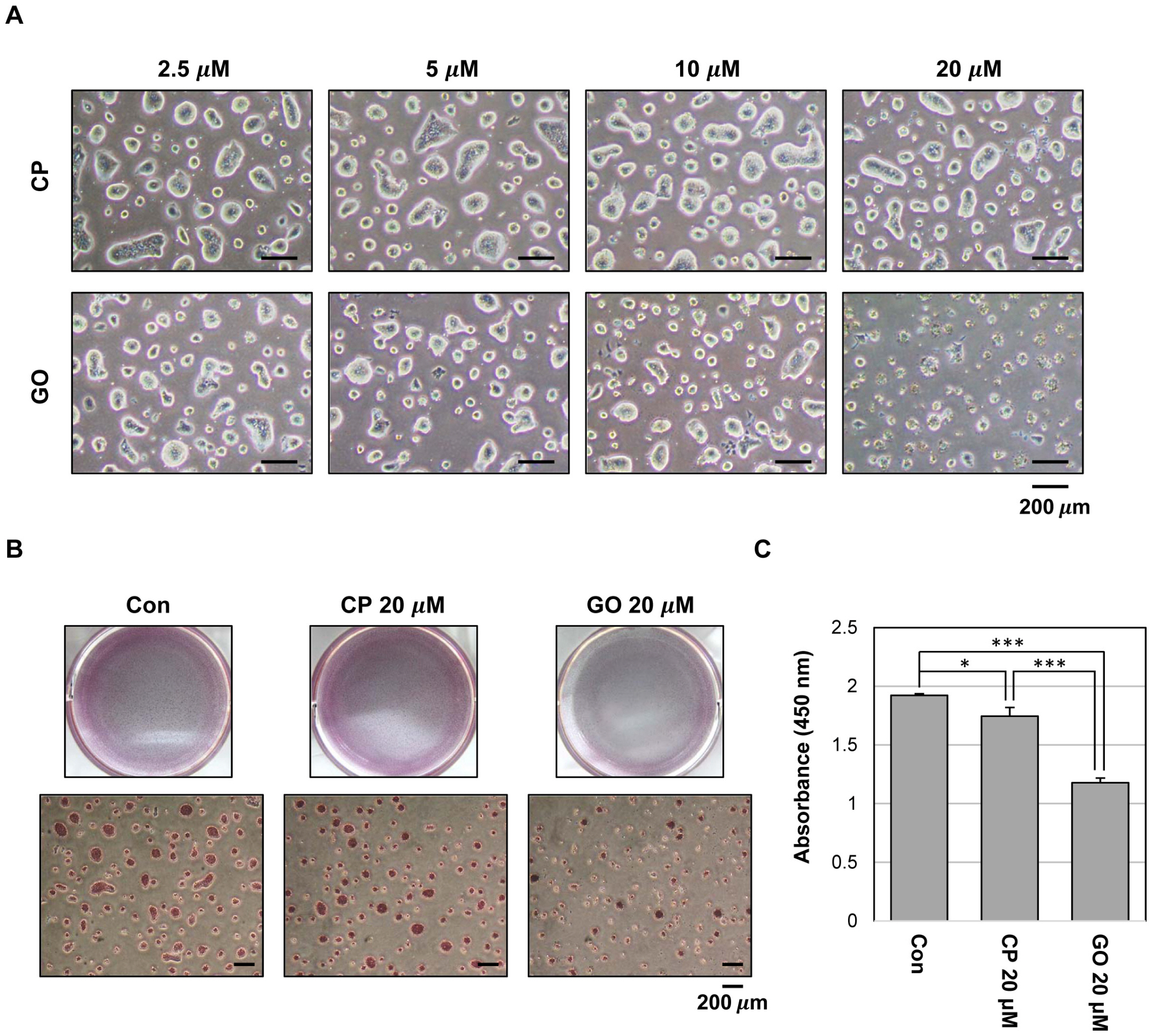
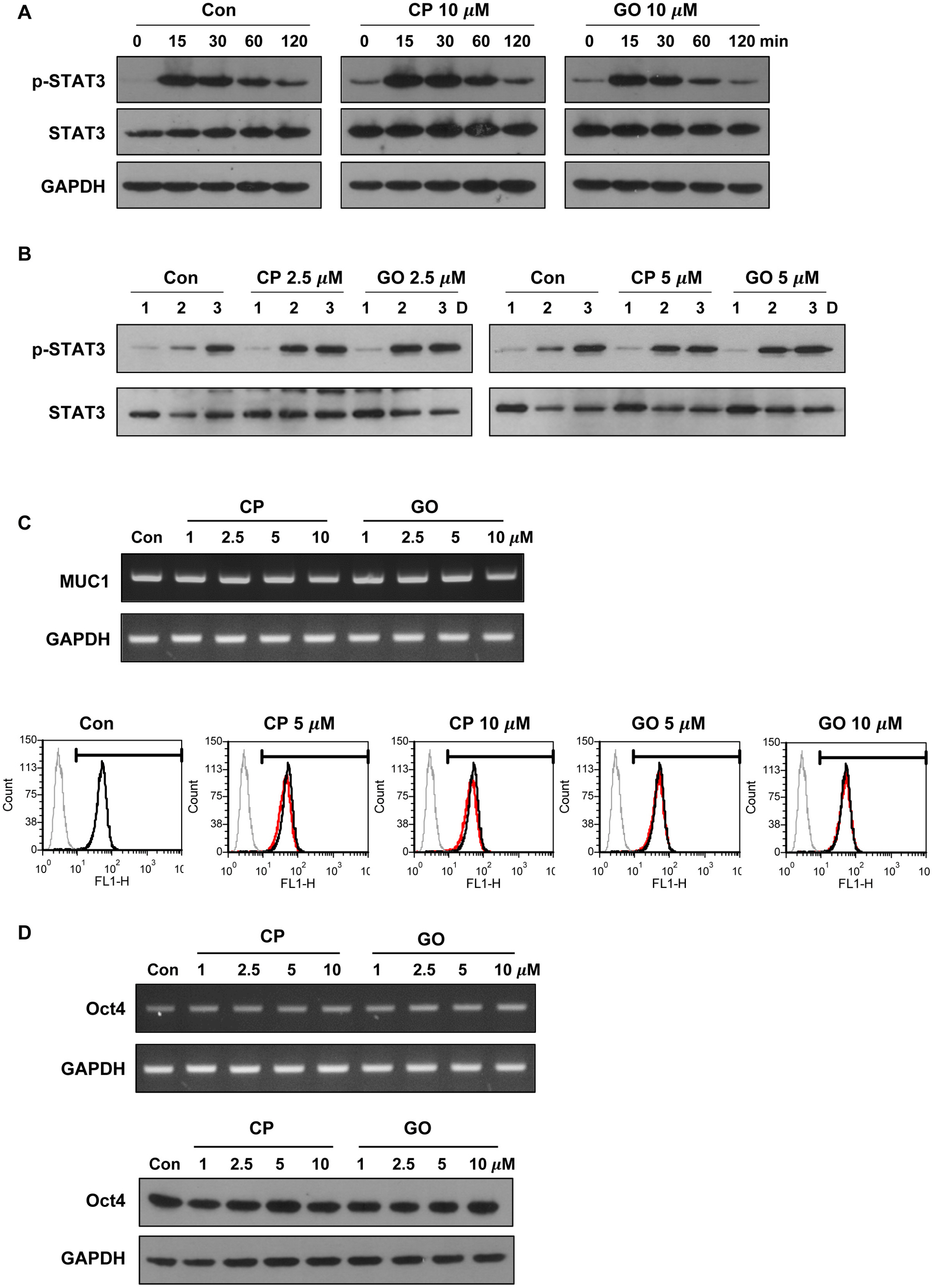
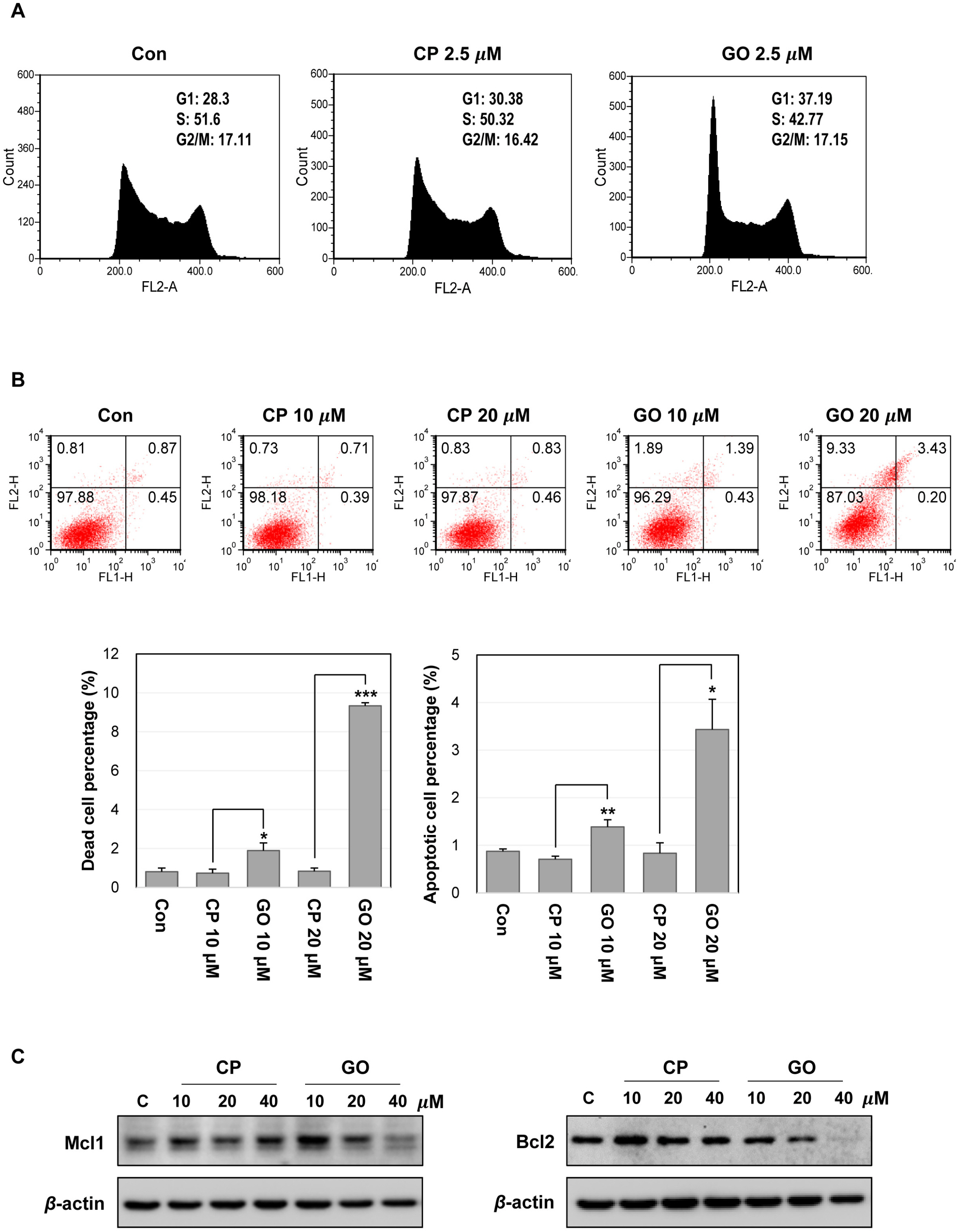
Undifferentiated mES cells expressed the MUC1-C protein and the expression level was decreased during differentiation. Inhibition of MUC1-C, by the specific inhibitor GO201, reduced proliferation of mES cells. However, there was no prominent effect on pluripotent markers such as Oct4 expression and STAT3 signaling, and MUC1-C inhibition did not induce differentiation. Inhibition of MUC1-C increased the G1 phase population, decreased the S phase population, and increased cell death. Furthermore, inhibition of MUC1-C induced disruption of the ROS balance in mES cells.
Conclusions
These results suggest that MUC1-C is involved in the growth and survival of mES cells.
Keyword
Figure
Reference
-
References
1. Evans MJ, Kaufman MH. 1981; Establishment in culture of pluripotential cells from mouse embryos. Nature. 292:154–156. DOI: 10.1038/292154a0. PMID: 7242681.
Article2. Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. 2004; Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 52:794–804. PMID: 15909857.3. Wang K, Zhang T, Dong Q, Nice EC, Huang C, Wei Y. 2013; Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 4:e537. DOI: 10.1038/cddis.2013.50. PMID: 23492768. PMCID: PMC3613828.
Article4. Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, Egashira T, Seki T, Muraoka N, Yamakawa H, Ohgino Y, Tanaka T, Yoichi M, Yuasa S, Murata M, Suematsu M, Fukuda K. 2013; Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 12:127–137. DOI: 10.1016/j.stem.2012.09.013. PMID: 23168164.
Article5. Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. 2012; Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 11:589–595. DOI: 10.1016/j.stem.2012.10.005. PMID: 23122286. PMCID: PMC3492890.
Article6. Folmes CD, Dzeja PP, Nelson TJ, Terzic A. 2012; Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 11:596–606. DOI: 10.1016/j.stem.2012.10.002. PMID: 23122287. PMCID: PMC3593051.
Article7. Saretzki G, Armstrong L, Leake A, Lako M, von Zglinicki T. 2004; Stress defense in murine embryonic stem cells is superior to that of various differentiated murine cells. Stem Cells. 22:962–971. DOI: 10.1634/stemcells.22-6-962. PMID: 15536187.
Article8. Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S, Stojkovic M, Armstrong L, von Zglinicki T, Lako M. 2008; Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 26:455–464. DOI: 10.1634/stemcells.2007-0628. PMID: 18055443.
Article9. Hollingsworth MA, Swanson BJ. 2004; Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 4:45–60. DOI: 10.1038/nrc1251. PMID: 14681689.
Article10. Kufe DW. 2009; Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 9:874–885. DOI: 10.1038/nrc2761. PMID: 19935676. PMCID: PMC2951677.
Article11. Ahmad R, Rajabi H, Kosugi M, Joshi MD, Alam M, Vasir B, Kawano T, Kharbanda S, Kufe D. 2011; MUC1-C oncoprotein promotes STAT3 activation in an autoinductive regulatory loop. Sci Signal. 4:ra9. DOI: 10.1126/scisignal.2001426. PMID: 21325207. PMCID: PMC3070357.
Article12. Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. 2005; MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 65:10413–10422. DOI: 10.1158/0008-5472.CAN-05-2474. PMID: 16288032.
Article13. Ahmad R, Raina D, Joshi MD, Kawano T, Ren J, Kharbanda S, Kufe D. 2009; MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor. Cancer Res. 69:7013–7021. DOI: 10.1158/0008-5472.CAN-09-0523. PMID: 19706766. PMCID: PMC2760979.
Article14. Niwa H, Burdon T, Chambers I, Smith A. 1998; Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12:2048–2060. DOI: 10.1101/gad.12.13.2048. PMID: 9649508. PMCID: PMC316954.
Article15. Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. 2008; The ground state of embryonic stem cell self-renewal. Nature. 453:519–523. DOI: 10.1038/nature06968. PMID: 18497825. PMCID: PMC5328678.
Article16. Kim YE, Kang HB, Park JA, Nam KH, Kwon HJ, Lee Y. 2008; Upregulation of NF-kappaB upon differentiation of mouse embryonic stem cells. BMB Rep. 41:705–709. DOI: 10.5483/BMBRep.2008.41.10.705. PMID: 18959816.17. Raina D, Ahmad R, Joshi MD, Yin L, Wu Z, Kawano T, Vasir B, Avigan D, Kharbanda S, Kufe D. 2009; Direct targeting of the mucin 1 oncoprotein blocks survival and tumori-genicity of human breast carcinoma cells. Cancer Res. 69:5133–5141. DOI: 10.1158/0008-5472.CAN-09-0854. PMID: 19491255. PMCID: PMC2721222.
Article18. Park JA, Kim YE, Ha YH, Kwon HJ, Lee Y. 2012; High sensitivity of embryonic stem cells to proteasome inhibitors correlates with low expression of heat shock protein and decrease of pluripotent cell marker expression. BMB Rep. 45:299–304. DOI: 10.5483/BMBRep.2012.45.5.299. PMID: 22617454.
Article19. Stavridis MP, Collins BJ, Storey KG. 2010; Retinoic acid orchestrates fibroblast growth factor signalling to drive embryonic stem cell differentiation. Development. 137:881–890. DOI: 10.1242/dev.043117. PMID: 20179094. PMCID: PMC2834455.
Article20. Raina D, Kosugi M, Ahmad R, Panchamoorthy G, Rajabi H, Alam M, Shimamura T, Shapiro GI, Supko J, Kharbanda S, Kufe D. 2011; Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Ther. 10:806–816. DOI: 10.1158/1535-7163.MCT-10-1050. PMID: 21421804. PMCID: PMC3092019.
Article21. White J, Dalton S. 2005; Cell cycle control of embryonic stem cells. Stem Cell Rev. 1:131–138. DOI: 10.1385/SCR:1:2:131. PMID: 17142847.
Article22. Yin L, Huang L, Kufe D. 2004; MUC1 oncoprotein activates the FOXO3a transcription factor in a survival response to oxidative stress. J Biol Chem. 279:45721–45727. DOI: 10.1074/jbc.M408027200. PMID: 15322085.
Article23. Higashi M, Yonezawa S, Ho JJ, Tanaka S, Irimura T, Kim YS, Sato E. 1999; Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology. 30:1347–1355. DOI: 10.1002/hep.510300609. PMID: 10573510.
Article24. Tamada S, Goto M, Nomoto M, Nagata K, Shimizu T, Tanaka S, Sakoda K, Imai K, Yonezawa S. 2002; Expression of MUC1 and MUC2 mucins in extrahepatic bile duct carcinomas: its relationship with tumor progression and prognosis. Pathol Int. 52:713–723. DOI: 10.1046/j.1440-1827.2002.01414.x. PMID: 12685548.
Article25. Singh AP, Chauhan SC, Bafna S, Johansson SL, Smith LM, Moniaux N, Lin MF, Batra SK. 2006; Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate. 66:421–429. DOI: 10.1002/pros.20372. PMID: 16302265.
Article26. Yonezawa S, Goto M, Yamada N, Higashi M, Nomoto M. 2008; Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics. 8:3329–3341. DOI: 10.1002/pmic.200800040. PMID: 18651706.
Article27. Jeon JM, Lee HW, Park JY, Jung HR, Hwang I, Kwon SY, Choe MS, Kang YN, Kim SP, Lee SS, Choi WI, Kwon KY. 2010; Expression of MUC1 and MUC4 and its prognostic significance in non-small cell lung carcinoma. Korean J Pathol. 44:397–403. DOI: 10.4132/KoreanJPathol.2010.44.4.397.
Article28. Jonckheere N, Van Seuningen I. 2010; The membrane-bound mucins: from cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie. 92:1–11. DOI: 10.1016/j.biochi.2009.09.018. PMID: 19818375.
Article29. Hikita ST, Kosik KS, Clegg DO, Bamdad C. 2008; MUC1* mediates the growth of human pluripotent stem cells. PLoS One. 3:e3312. DOI: 10.1371/journal.pone.0003312. PMID: 18833326. PMCID: PMC2553196.
Article30. Spicer AP, Parry G, Patton S, Gendler SJ. 1991; Molecular cloning and analysis of the mouse homologue of the tumor-associated mucin, MUC1, reveals conservation of potential O-glycosylation sites, transmembrane, and cytoplasmic domains and a loss of minisatellite-like polymorphism. J Biol Chem. 266:15099–15109. DOI: 10.1016/S0021-9258(18)98592-3. PMID: 1714452.
Article31. Wei X, Xu H, Kufe D. 2007; Human mucin 1 oncoprotein represses transcription of the p53 tumor suppressor gene. Cancer Res. 67:1853–1858. DOI: 10.1158/0008-5472.CAN-06-3063. PMID: 17308127.
Article32. Lillehoj EP, Lu W, Kiser T, Goldblum SE, Kim KC. 2007; MUC1 inhibits cell proliferation by a beta-catenin-dependent mechanism. Biochim Biophys Acta. 1773:1028–1038. DOI: 10.1016/j.bbamcr.2007.04.009. PMID: 17524503. PMCID: PMC2349984.33. Niwa H, Miyazaki J, Smith AG. 2000; Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 24:372–376. DOI: 10.1038/74199. PMID: 10742100.
Article34. Wei X, Xu H, Kufe D. 2005; Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 7:167–178. DOI: 10.1016/j.ccr.2005.01.008. PMID: 15710329.
Article35. Yin L, Kharbanda S, Kufe D. 2009; MUC1 oncoprotein promotes autophagy in a survival response to glucose deprivation. Int J Oncol. 34:1691–1699. DOI: 10.3892/ijo_00000300. PMID: 19424588. PMCID: PMC3027209.
Article36. Yin L, Li Y, Ren J, Kuwahara H, Kufe D. 2003; Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem. 278:35458–35464. DOI: 10.1074/jbc.M301987200. PMID: 12826677.
Article37. Kosugi M, Ahmad R, Alam M, Uchida Y, Kufe D. 2011; MUC1-C oncoprotein regulates glycolysis and pyruvate kinase M2 activity in cancer cells. PLoS One. 6:e28234. DOI: 10.1371/journal.pone.0028234. PMID: 22140559. PMCID: PMC3225393.
Article38. Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X, Zhang B, Mehla K, Brown RB, Caffrey T, Yu F, Johnson KR, Powers R, Hollingsworth MA, Singh PK. 2012; MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci U S A. 109:13787–13792. DOI: 10.1073/pnas.1203339109. PMID: 22869720. PMCID: PMC3427054.
Article39. Kim H, Jang H, Kim TW, Kang BH, Lee SE, Jeon YK, Chung DH, Choi J, Shin J, Cho EJ, Youn HD. 2015; Core pluripotency factors directly regulate metabolism in embryonic stem cell to maintain pluripotency. Stem Cells. 33:2699–2711. DOI: 10.1002/stem.2073. PMID: 26059508.
Article