Korean Circ J.
2021 May;51(5):455-468. 10.4070/kcj.2020.0415.
Ventricular Arrhythmia Burden as a Marker of Success Following Catheter Ablation of Ventricular Arrhythmias in Patients with Structural Heart Disease
- Affiliations
-
- 1Department of Cardiology, Westmead Hospital, The University of Sydney, Westmead, Australia
- KMID: 2515772
- DOI: http://doi.org/10.4070/kcj.2020.0415
Abstract
- Background and Objectives
There is little emphasis on the efficacy of catheter ablation for ventricular arrhythmia (VA) when using VA burden reduction as a marker for success. We examined the efficacy of catheter ablation using VA burden, rather than VA recurrence as a marker of success, following catheter ablation of structural heart disease (SHD) related VA.
Methods
Catheter ablation of SHD related VA was performed at a single centre over 4-years. VA episodes and implantable cardioverter defibrillator (ICD) therapies were recorded over the 6-months before and after final ablation. Outcomes were reported in terms of burden reduction and compared to singular VA recurrence.
Results
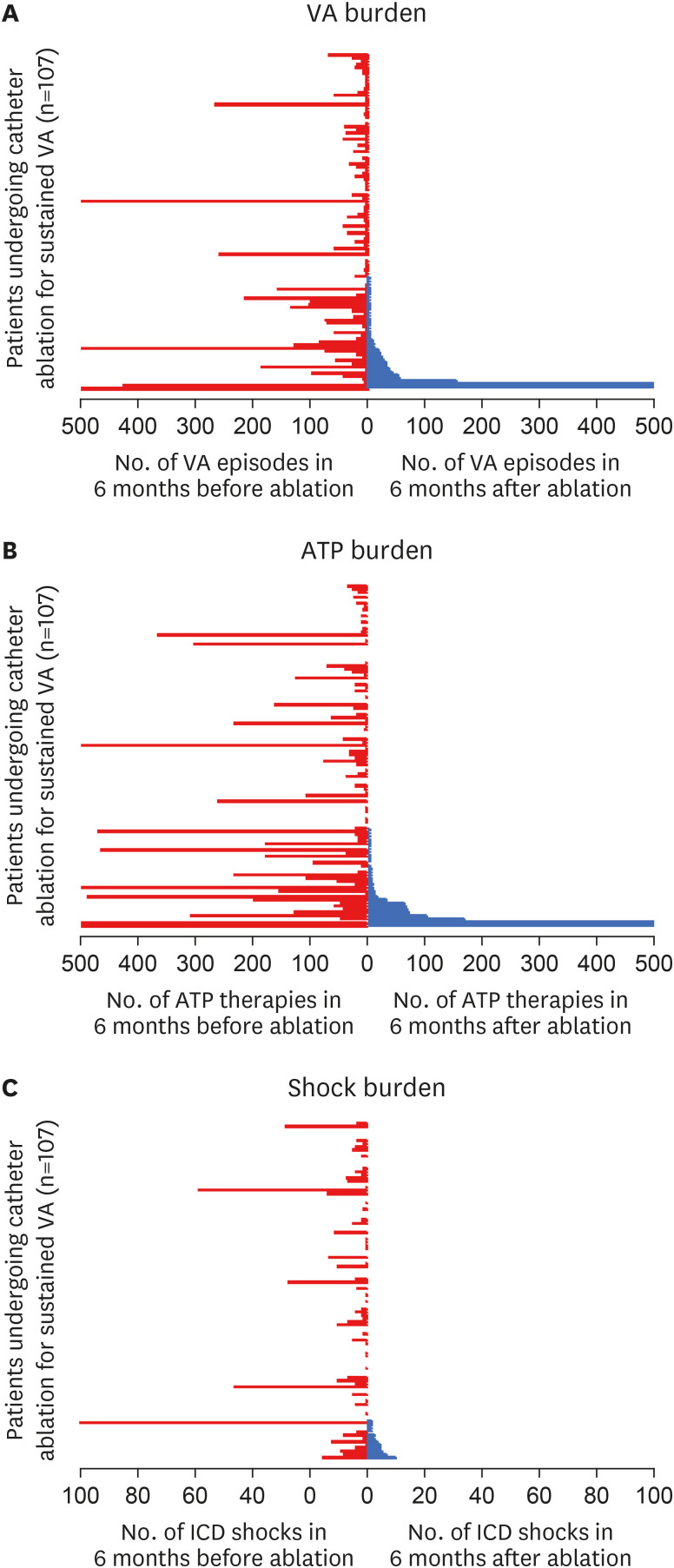
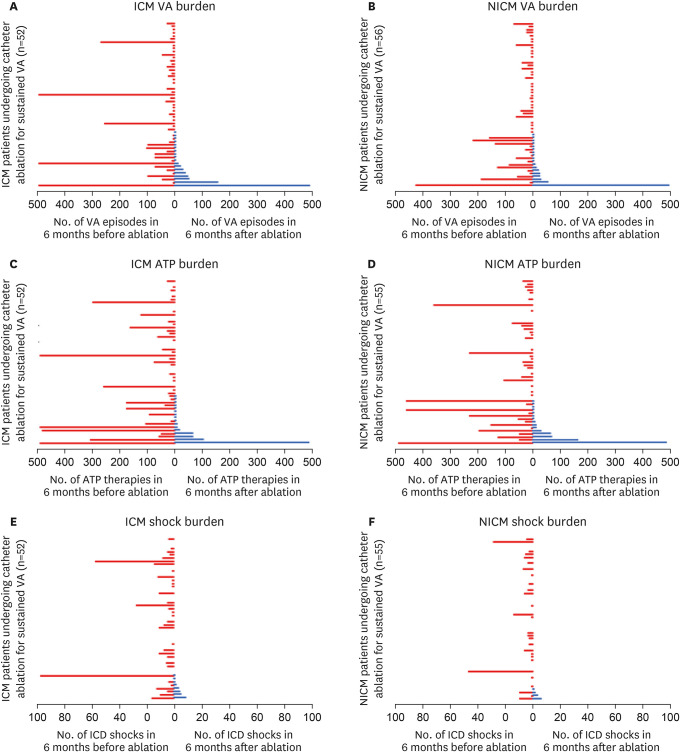
Overall, 108 patients were included in the study. Mean age 64.2±13.9 years, 86% male, mean left ventricular ejection fraction (LVEF) 42±16%. Median VA episodes and ICD therapy were significantly reduced after ablation (VA before: 10 [interquartile range, IQR: 2–38] vs. VA after: 0 [IQR: 0–2], p<0.001; anti–tachycardia pacing [ATP] before: 16 (IQR: 1.5– 57) vs. ATP after: 0 [IQR: 0–2], p<0.001; shocks before: 1 [IQR: 0–5] vs. shocks after: 0 [IQR: 0–0], p<0.001). Procedural success at 6-months was significantly higher when considering ≥75% reduction in VA burden, rather than a singular VA-free survival (83% vs. 67%, p=0.001).
Conclusions
The vast majority (>80%) of patients achieve reduction in VA burden (≥75% reduction) after catheter ablation for VA. This data suggests that catheter ablation is highly therapeutic when procedure success is defined as reduction in VA, rather than using a single VA recurrence as a metric for failure.
Keyword
Figure
Cited by 1 articles
-
Meaning of Ventricular Arrhythmia Burden Reduction as a Marker of Ablation Success
Namsik Yoon
Korean Circ J. 2021;51(5):469-470. doi: 10.4070/kcj.2021.0069.
Reference
-
1. Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: executive summary. J Arrhythm. 2020; 36:1–58. PMID: 32071620.2. Sun S, Johnson J, Degroot P, Brown ML, Obel O. Effect of ICD therapies on mortality in the OMNI trial. J Cardiovasc Electrophysiol. 2016; 27:192–199. PMID: 26501695.
Article3. Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008; 359:1009–1017. PMID: 18768944.
Article4. Kumar S, Romero J, Mehta NK, et al. Long-term outcomes after catheter ablation of ventricular tachycardia in patients with and without structural heart disease. Heart Rhythm. 2016; 13:1957–1963. PMID: 27392945.
Article5. Marchlinski FE, Haffajee CI, Beshai JF, et al. Long-term success of irrigated radiofrequency catheter ablation of sustained ventricular tachycardia: post-approval THERMOCOOL VT trial. J Am Coll Cardiol. 2016; 67:674–683. PMID: 26868693.6. Cuculich PS, Schill MR, Kashani R, et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017; 377:2325–2336. PMID: 29236642.
Article7. Armaganijan LV, Staico R, Moreira DA, et al. 6-month outcomes in patients with implantable cardioverter-defibrillators undergoing renal sympathetic denervation for the treatment of refractory ventricular arrhythmias. JACC Cardiovasc Interv. 2015; 8:984–990. PMID: 26088516.
Article8. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008; 29:270–276. PMID: 17916581.
Article9. Campbell T, Trivic I, Bennett RG, et al. Catheter ablation of ventricular arrhythmia guided by a high-density grid catheter. J Cardiovasc Electrophysiol. 2020; 31:474–484. PMID: 31930658.
Article10. Anderson RD, Lee G, Trivic I, et al. Focal ventricular tachycardias in structural heart disease: prevalence, characteristics, and clinical outcomes after catheter ablation. JACC Clin Electrophysiol. 2020; 6:56–69. PMID: 31971907.11. Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000; 101:1288–1296. PMID: 10725289.
Article12. Hutchinson MD, Gerstenfeld EP, Desjardins B, et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011; 4:49–55. PMID: 21131557.
Article13. Polin GM, Haqqani H, Tzou W, et al. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011; 8:76–83. PMID: 20933099.
Article14. Kumar S, Barbhaiya C, Nagashima K, et al. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol. 2015; 8:87–93. PMID: 25527825.15. Andrade JG, Champagne J, Dubuc M, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019; 140:1779–1788. PMID: 31630538.16. Yokoyama K, Nakagawa H, Shah DC, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008; 1:354–362. PMID: 19808430.
Article17. Robinson CG, Samson PP, Moore KMS, et al. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 2019; 139:313–321. PMID: 30586734.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Updates in Ventricular Tachycardia Ablation
- Catheter Ablation of Ventricular Arrhythmias via the Radial Artery in a Patient With Prior Myocardial Infarction and Peripheral Vascular Disease
- Diagnosis and Treatment of Premature Atrial or Ventricular Complexes
- 2018 KHRS Guidelines for Catheter Ablation of Ventricular Arrhythmias: Part 3
- Catheter ablation for treatment of tachyarrhythmia