J Korean Neurosurg Soc.
2021 May;64(3):367-373. 10.3340/jkns.2021.0054.
Cell Lineage, Self-Renewal, and Epithelial-to-Mesenchymal Transition during Secondary Neurulation
- Affiliations
-
- 1Department of Zoology, Graduate School of Science, Kyoto University, Kyoto, Japan
- 2Faculty of Engineering, Okayama University of Science, Okayama, Japan
- KMID: 2515492
- DOI: http://doi.org/10.3340/jkns.2021.0054
Abstract
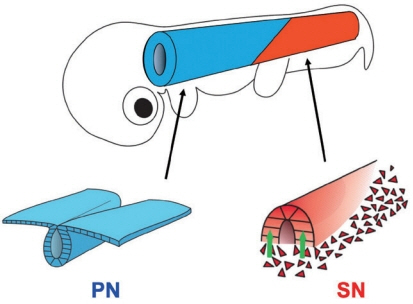
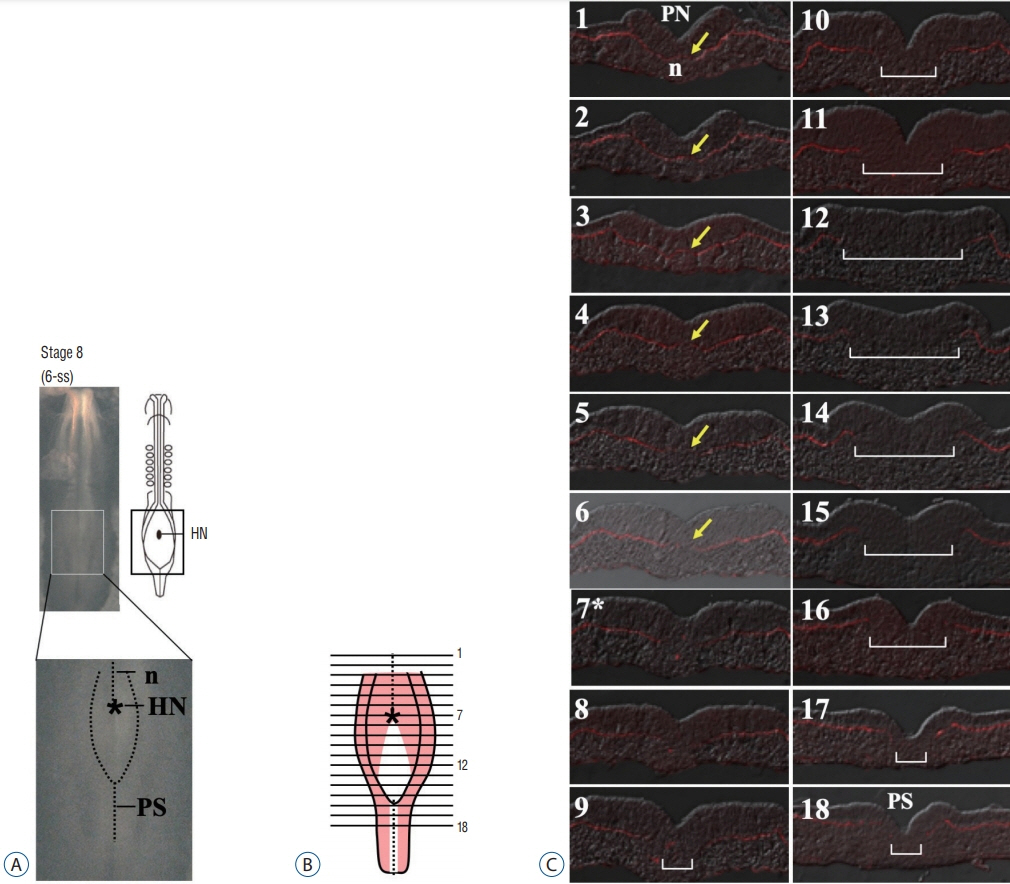
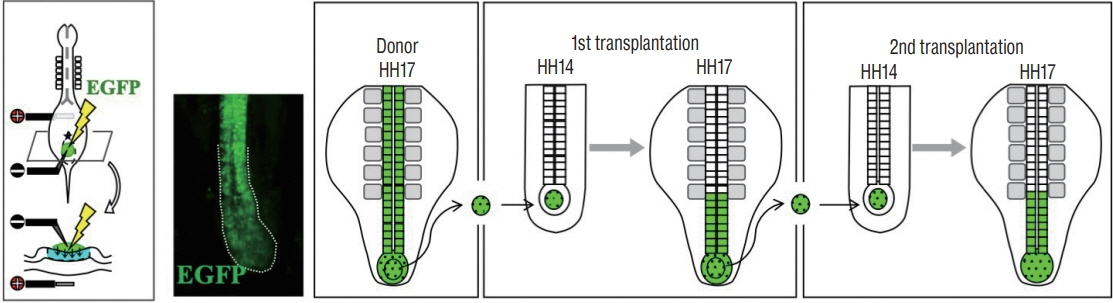
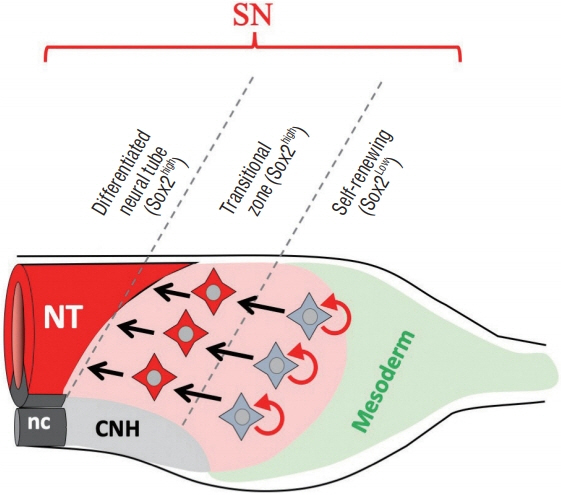
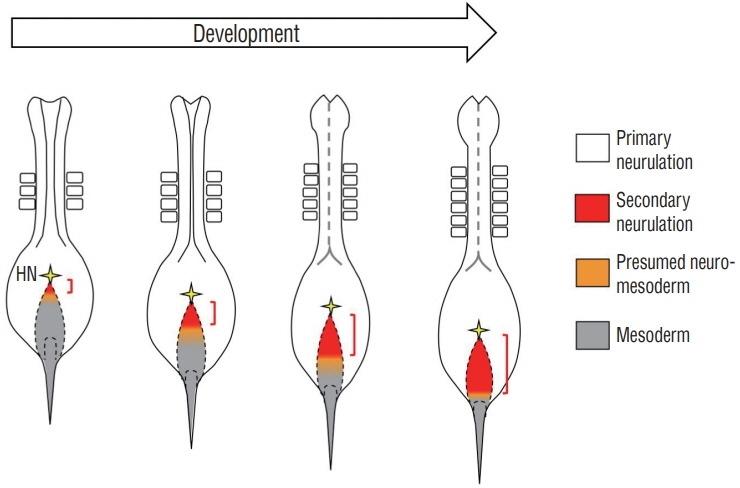
- Secondary neurulation (SN) is a critical process to form the neural tube in the posterior region of the body including the tail. SN is distinct from the anteriorly occurring primary neurulation (PN); whereas the PN proceeds by folding an epithelial neural plate, SN precursors arise from a specified epiblast by epithelial-to-mesenchymal transition (EMT), and undergo self-renewal in the tail bud. They finally differentiate into the neural tube through mesenchymal-to-epithelial transition (MET). We here overview recent progresses in the studies of SN with a particular focus on the regulation of cell lineage, self-renewal, and EMT/MET. Cellular mechanisms underlying SN help to understand the functional diversity of the tail in vertebrates.
Figure
Reference
-
References
1. Catala M. Genetic control of caudal development. Clin Genet. 61:89–96. 2002.
Article2. Catala M, Teillet MA, Le Douarin NM. Organization and development of the tail bud analyzed with the quail-chick chimaera system. Mech Dev. 51:51–65. 1995.
Article3. Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 221:117–145. 2001.
Article4. Criley BB. Analysis of embryonic sources and mechanims of development of posterior levels of chick neural tubes. J Morphol. 128:465–501. 1969.
Article5. Dady A, Havis E, Escriou V, Catala M, Duband JL. Junctional neurulation: a unique developmental program shaping a discrete region of the spinal cord highly susceptible to neural tube defects. J Neurosci. 34:13208–13221. 2014.
Article6. Garcia-Martinez V, Darnell DK, Lopez-Sanchez C, Sosic D, Olson EN, Schoenwolf GC. State of commitment of prospective neural plate and prospective mesoderm in late gastrula/early neurula stages of avian embryos. Dev Biol. 181:102–115. 1997.
Article7. Gouti M, Delile J, Stamataki D, Wymeersch FJ, Huang Y, Kleinjung J, et al. A gene regulatory network balances neural and mesoderm specification during vertebrate trunk development. Dev Cell. 41:243–261.e7. 2017.
Article8. Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, et al. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 12:e1001937. 2014.
Article9. Griffith CM, Wiley MJ, Sanders EJ. The vertebrate tail bud: three germ layers from one tissue. Anat Embryol (Berl). 185:101–113. 1992.
Article10. Guillot C, Michaut A, Rabe B, Pourquié O. Dynamics of primitive streak regression controls the fate of neuro-mesodermal progenitors in the chicken embryo. bioRxiv. 2020; [Epub ahead of print].
Article11. Holmdahl DE. Die Morphogenese des Vertebratorganismus vom formalen und experimentellen Gesichtspunkt. W Roux’ Archiv f Entwicklungsmechanik. 139:191–226. 1939.
Article12. Iimura T, Pourquié O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 442:568–571. 2006.
Article13. Kardong KV. Vertebrates: Comparative Anatomy, Function, Evolution. 4th ed. New York: McGraw-Hill College;2005.14. Kawachi T, Shimokita E, Kudo R, Tadokoro R, Takahashi Y. Neural-fated self-renewing cells regulated by Sox2 during secondary neurulation in chicken tail bud. Dev Biol. 461:160–171. 2020.
Article15. Nievelstein RA, Hartwig NG, Vermeij-Keers C, Valk J. Embryonic development of the mammalian caudal neural tube. Teratology. 48:21–31. 1993.
Article16. Nikolopoulou E, Galea GL, Rolo A, Greene ND, Copp AJ. Neural tube closure: cellular, molecular and biomechanical mechanisms. Development. 144:552–566. 2017.
Article17. Olivera-Martinez I, Harada H, Halley PA, Storey KG. Loss of FGF-dependent mesoderm identity and rise of endogenous retinoid signalling determine cessation of body axis elongation. PLoS Biol. 10:e1001415. 2012.
Article18. Pasteels J. Etudes sur la gastrulation des vertébrés méroblastiques. III. Oiseaux. IV Conclusions générales. Arch Biol. 48:381–488. 1937.19. Romanos M, Allio G, Combres L, Médevielle F, Escalas N, Soula C, et al. Cell-to-cell heterogeneity in Sox2 and Brachyury expression ratios guides progenitor destiny by controlling their motility. bioRxiv. 2020; [Epub ahead of print].
Article20. Saitsu H, Yamada S, Uwabe C, Ishibashi M, Shiota K. Development of the posterior neural tube in human embryos. Anat Embryol (Berl). 209:107–117. 2004.
Article21. Shaker MR, Lee JH, Kim KH, Kim JV, Kim JY, Lee JY, et al. Spatiotemporal contribution of neuromesodermal progenitor-derived neural cells in the elongation of developing mouse spinal cord. bioRxiv. 2020; [Epub ahead of print].
Article22. Shimokita E, Takahashi Y. Secondary neurulation: fate-mapping and gene manipulation of the neural tube in tail bud. Dev Growth Differ. 53:401–410. 2011.
Article23. Takemoto T, Uchikawa M, Yoshida M, Bell DM, Lovell-Badge R, Papaioannou VE, et al. Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature. 470:394–398. 2011.
Article24. Tucker AS, Slack JM. Tail bud determination in the vertebrate embryo. Curr Biol. 5:807–813. 1995.
Article25. Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell. 17:365–376. 2009.
Article26. Uchikawa M, Yoshida M, Iwafuchi-Doi M, Matsuda K, Ishida Y, Takemoto T, et al. B1 and B2 Sox gene expression during neural plate development in chicken and mouse embryos: universal versus speciesdependent features. Dev Growth Differ. 53:761–771. 2011.
Article27. Watanabe T, Saito D, Tanabe K, Suetsugu R, Nakaya Y, Nakagawa S, et al. Tet-on inducible system combined with in ovo electroporation dissects multiple roles of genes in somitogenesis of chicken embryos. Dev Biol. 305:625–636. 2007.
Article28. Wymeersch FJ, Huang Y, Blin G, Cambray N, Wilkie R, Wong FC, et al. Position-dependent plasticity of distinct progenitor types in the primitive streak. Elife. 5:e10042. 2016.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Perspectives : The Role of Clinicians in Understanding Secondary Neurulation
- Targeting epithelial-mesenchymal transition pathway in hepatocellular carcinoma
- Membrane Proteins Involved in Epithelial-Mesenchymal Transition and Tumor Invasion: Studies on TMPRSS4 and TM4SF5
- Disorders of Secondary Neurulation : Mainly Focused on Pathoembryogenesis
- Consideration of EphA2 in relation to epithelial-mesenchymal transition in uterine endometrial cancer