Intest Res.
2021 Apr;19(2):239-246. 10.5217/ir.2020.00009.
Postgastrectomy gastric cancer patients are at high risk for colorectal neoplasia: a case control study
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2515483
- DOI: http://doi.org/10.5217/ir.2020.00009
Abstract
- Background/Aims
Several studies have shown that colorectal neoplasms (CRN) including colorectal cancer (CRC) may be prevalent in patients with gastric cancer. However, in most of these studies, colonoscopy to investigate the prevalence of CRN was performed prior to surgery. We aimed to investigate whether CRN was more prevalent in postgastrectomy gastric cancer patients than in healthy individuals.
Methods
We reviewed the medical records of those patients within a cohort of gastric cancer patients with gastrectomy who underwent colonoscopy between 2016 and 2017. Controls age- and sex-matched with gastric cancer patients at a 2:1 ratio were identified among those who underwent colonoscopy at a health-promotion center. The frequencies of CRN, advanced CRN (ACRN), and CRC among patients with gastrectomy were compared with those in the control subjects. A total of 744 individuals (gastric cancer, 248; control, 496) were included.
Results
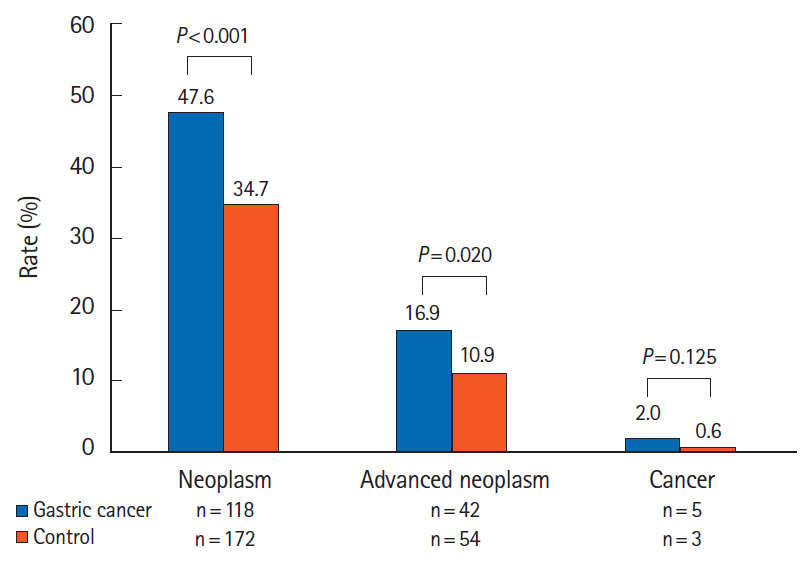
The rates of CRN and ACRN in the gastric cancer group were higher than those in the healthy individuals (CRN, 47.6% vs. 34.7%, P< 0.001; ACRN, 16.9% vs. 10.9%, P= 0.020). The rate of CRC was comparable between the 2 groups (2.0% vs. 0.6%, P= 0.125). Multivariate analysis identified previous gastrectomy for gastric cancer and male sex as significant risk factors for (A)CRN.
Conclusions
CRN and ACRN were more prevalent in patients who underwent surgery for gastric cancer than in the control group. Regular surveillance colonoscopy at appropriate intervals is indicated after gastrectomy.
Figure
Cited by 1 articles
-
Calcium, Vitamin D, and Colorectal Cancer
Young-Jo Wi, Soo-Young Na
Korean J Gastroenterol. 2023;82(2):47-55. doi: 10.4166/kjg.2023.091.
Reference
-
1. Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. 2017; 152:1319–1328.
Article2. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018; 391:1023–1075.3. Ikeda Y, Saku M, Kawanaka H, Nonaka M, Yoshida K. Features of second primary cancer in patients with gastric cancer. Oncology. 2003; 65:113–117.
Article4. Lee JH, Bae JS, Ryu KW, et al. Gastric cancer patients at high-risk of having synchronous cancer. World J Gastroenterol. 2006; 12:2588–2592.
Article5. Eom BW, Lee HJ, Yoo MW, et al. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008; 98:106–110.
Article6. Saito S, Hosoya Y, Togashi K, et al. Prevalence of synchronous colorectal neoplasms detected by colonoscopy in patients with gastric cancer. Surg Today. 2008; 38:20–25.
Article7. Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002; 89:845–860.
Article8. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012; 143:844–857.
Article9. Choi WS, Han DS, Eun CS, et al. Three-year colonoscopy surveillance after polypectomy in Korea: a Korean Association for the Study of Intestinal Diseases (KASID) multicenter prospective study. Intest Res. 2018; 16:126–133.
Article10. Gweon TG, Huh CW, Ji JS, Kim CH, Kim JJ, Park SM. Comparison of bowel-cleansing efficacy of split-dose and same-day dose bowel preparation for afternoon colonoscopy in patients with gastrectomy: a prospective randomized study. Surg Endosc. 2020; 34:4413–4421.
Article11. Yoo HM, Gweon TG, Seo HS, et al. Role of preoperative colonoscopy in patients with gastric cancer: a case control study of the prevalence of coexisting colorectal neoplasms. Ann Surg Oncol. 2013; 20:1614–1622.
Article12. Washington K. 7th Edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010; 17:3077–3079.
Article13. Huh CW, Gweon TG, Seo M, Ji JS, Kim BW, Choi H. Validation of same-day bowel preparation regimen using 4L polyethylene glycol: comparison of morning and afternoon colonoscopy. Medicine (Baltimore). 2018; 97:e12431.14. Seo M, Gweon TG, Huh CW, Ji JS, Choi H. Comparison of bowel cleansing efficacy, safety, bowel movement kinetics, and patient tolerability of same-day and split-dose bowel preparation using 4 L of polyethylene glycol: a prospective randomized study. Dis Colon Rectum. 2019; 62:1518–1527.
Article15. Lee HS, Byeon JS. Bowel preparation, the first step for a good quality colonoscopy. Intest Res. 2014; 12:1–2.
Article16. Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000; 52:346–352.
Article17. Gweon TG, Kim SW, Noh YS, et al. Prospective, randomized comparison of same-day dose of 2 different bowel cleanser for afternoon colonoscopy: picosulfate, magnesium oxide, and citric acid versus polyethylene glycol. Medicine (Baltimore). 2015; 94:e628.18. European Colorectal Cancer Screening Guidelines Working Group, von Karsa L, Patnick J, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013; 45:51–59.
Article19. Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006; 355:2533–2541.
Article20. Kim SY, Kim TI. Serrated neoplasia pathway as an alternative route of colorectal cancer carcinogenesis. Intest Res. 2018; 16:358–365.
Article21. Ferrari P, Jenab M, Norat T, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer. 2007; 121:2065–2072.
Article22. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010; 138:2029–2043.
Article23. Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016; 150:1113–1124.
Article24. Zumkeller N, Brenner H, Zwahlen M, Rothenbacher D. Helicobacter pylori infection and colorectal cancer risk: a metaanalysis. Helicobacter. 2006; 11:75–80.
Article25. Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012; 366:697–706.
Article26. Papastergiou V, Paraskeva KD, Fragaki M, et al. Cold versus hot endoscopic mucosal resection for nonpedunculated colorectal polyps sized 6-10 mm: a randomized trial. Endoscopy. 2018; 50:403–411.
Article27. Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006; 355:1863–1872.
Article28. Jaruvongvanich V, Sempokuya T, Laoveeravat P, Ungprasert P. Risk factors associated with longer cecal intubation time: a systematic review and meta-analysis. Int J Colorectal Dis. 2018; 33:359–365.
Article29. Park JM, Huo SM, Lee HH, Lee BI, Song HJ, Choi MG. Longer observation time increases proportion of neoplasms detected by esophagogastroduodenoscopy. Gastroenterology. 2017; 153:460–469.
Article30. Woo DH, Kim KO, Jeong DE, et al. Prospective analysis of factors associated with inadequate bowel preparation for colonoscopy in actual clinical practice. Intest Res. 2018; 16:293–298.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of Dietary Vitamin D and Calcium With Genetic Polymorphisms in Colorectal Neoplasia
- Prevalence and associated risk of advanced colorectal neoplasia in adults with sarcopenia
- Strategies for colorectal cancer screening and post-polypectomy surveillance for young adults under age 50
- Histological inflammation increases the risk of colorectal neoplasia in ulcerative colitis: a systematic review
- Comparison of Colonoscopy Surveillance Outcomes Between Young and Older Colorectal Cancer Patients