Endocrinol Metab.
2021 Apr;36(2):270-278. 10.3803/EnM.2021.970.
Operationalizing Treat-to-Target for Osteoporosis
- Affiliations
-
- 1New Mexico Clinical Research & Osteoporosis Center, Albuquerque, NM, USA
- KMID: 2515454
- DOI: http://doi.org/10.3803/EnM.2021.970
Abstract
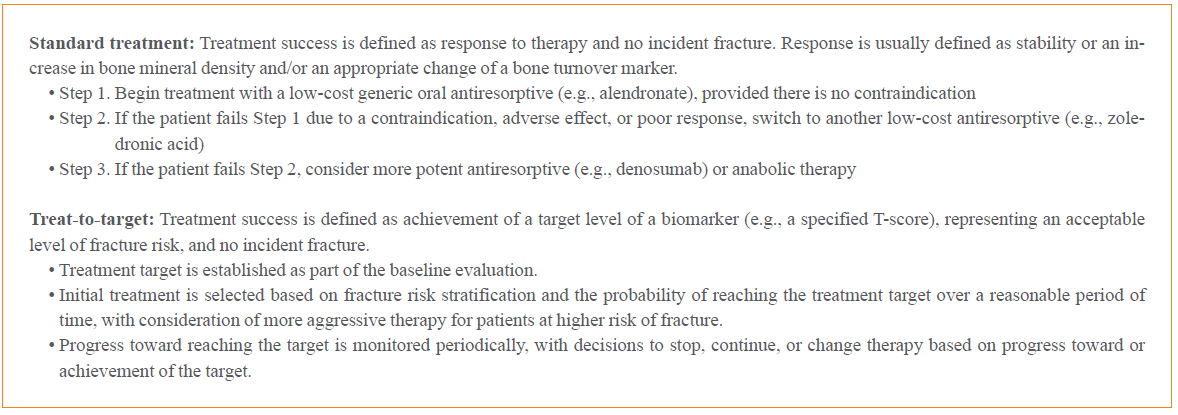
- Treat-to-target (TTT) for osteoporosis is a concept for individualizing patient treatment decisions that focuses on achieving an acceptable level of fracture risk rather than response to treatment alone. While a response to treatment is essential in order to achieve an acceptable level of risk, it is not necessarily sufficient. Some patients have a good response to treatment yet remain at high level of fracture risk. Since there is no way to directly measure bone strength in patients treated for osteoporosis, a surrogate measurement must be used. Bone mineral density (BMD) is commonly used to select patients for treatment and has emerged as the most useful surrogate for assessing reduction of fracture risk after treatment is started. Recent large meta-regression studies have shown a robust correlation between larger increases in BMD with treatment and greater reductions in fracture risk. Application of TTT for osteoporosis involves assessing fracture risk before starting treatment and initiating treatment with an agent that is most likely to reduce fracture risk to an acceptable level, represented by a target BMD T-score, over a reasonable period of time. This review offers suggestions for implementing TTT for osteoporosis in clinical practice and managing patients who fail or succeed in reaching the target. More study is needed to fully validate the use of TTT for osteoporosis for initiating and modifying treatments to reduce fracture risk.
Keyword
Figure
Reference
-
1. Verdecchia P, Reboldi G, Angeli F. The 2020 International Society of Hypertension global hypertension practice guidelines: key messages and clinical considerations. Eur J Intern Med. 2020; 82:1–6.
Article2. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020; 43(Suppl 1):S66–76.3. Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020; 79:685–99.4. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001; 285:785–95.5. Khosla S, Bilezikian JP, Dempster DW, Lewiecki EM, Miller PD, Neer RM, et al. Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metab. 2012; 97:2272–82.
Article6. Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009; 24:153–61.
Article7. Brown JP, Roux C, Ho PR, Bolognese MA, Hall J, Bone HG, et al. Denosumab significantly increases bone mineral density and reduces bone turnover compared with monthly oral ibandronate and risedronate in postmenopausal women who remained at higher risk for fracture despite previous suboptimal treatment with an oral bisphosphonate. Osteoporos Int. 2014; 25:1953–61.
Article8. Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab. 2016; 101:3163–70.
Article9. Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017; 377:1417–27.
Article10. Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007; 357:2028–39.
Article11. Hadji P, Zanchetta JR, Russo L, Recknor CP, Saag KG, McKiernan FE, et al. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int. 2012; 23:2141–50.
Article12. Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018; 391:230–40.
Article13. Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016; 316:722–33.
Article14. Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017; 390:1585–94.
Article15. Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, et al. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019; 34:632–42.
Article16. Black DM, Bauer DC, Vittinghoff E, Lui LY, Grauer A, Marin F, et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020; 8:672–82.
Article17. Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017; 32:198–202.
Article18. Cummings SR, Cosman F, Eastell R, Reid IR, Mehta M, Lewiecki EM. Goal-directed treatment of osteoporosis. J Bone Miner Res. 2013; 28:433–8.
Article19. Lewiecki EM, Cummings SR, Cosman F. Treat-to-target for osteoporosis: is now the time? J Clin Endocrinol Metab. 2013; 98:946–53.
Article20. Kanis JA, McCloskey E, Branco J, Brandi ML, Dennison E, Devogelaer JP, et al. Goal-directed treatment of osteoporosis in Europe. Osteoporos Int. 2014; 25:2533–43.
Article21. Miedany YE. Treat to target for osteoporosis: another step forward. Curr Rheumatol Rev. 2014; 10:99–105.
Article22. Cummings SR, Cosman F, Lewiecki EM, Schousboe JT, Bauer DC, Black DM, et al. Goal-directed treatment for osteoporosis: a progress report from the ASBMR-NOF working group on goal-directed treatment for osteoporosis. J Bone Miner Res. 2017; 32:3–10.
Article23. Lewiecki EM, Watts NB. Assessing response to osteoporosis therapy. Osteoporos Int. 2021; In Press.
Article24. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2021; In press.25. Thomas T, Casado E, Geusens P, Lems WF, Timoshanko J, Taylor D, et al. Is a treat-to-target strategy in osteoporosis applicable in clinical practice? Consensus among a panel of European experts. Osteoporos Int. 2020; 31:2303–11.
Article26. Nogues X, Nolla JM, Casado E, Jodar E, Munoz-Torres M, Quesada-Gomez JM, et al. Spanish consensus on treat to target for osteoporosis. Osteoporos Int. 2018; 29:489–99.27. Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, et al. Treatment failure in osteoporosis. Osteoporos Int. 2012; 23:2769–74.
Article28. Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab. 2000; 85:231–6.
Article29. Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002; 112:281–9.
Article30. Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002; 87:1586–92.
Article31. Lewiecki EM, Kendler DL, Davison KS, Hanley DA, Harris ST, McClung MR, et al. Western osteoporosis alliance clinical practice series: treat-to-target for osteoporosis. Am J Med. 2019; 132:e771–7.
Article32. Ferrari S, Libanati C, Lin CJF, Brown JP, Cosman F, Czerwinski E, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019; 34:1033–40.
Article33. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019; 104:1595–622.
Article34. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020; 105:dgaa048.
Article35. Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis: 2020 update. Endocr Pract. 2020; 26(Suppl 1):1–46.
Article36. Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guanabens N, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017; 105:11–7.
Article37. Lewiecki EM. Marcus and Feldman’s osteoporosis. 5th ed. San Diego: Elsevier;2021. Chapter 61:Evaluation of the osteoporosis patient. p. 1475–500.38. Lewiecki EM, Laster AJ. Clinical review: clinical applications of vertebral fracture assessment by dual-energy X-ray absorptiometry. J Clin Endocrinol Metab. 2006; 91:4215–22.39. Borges JLC, Sousa da Silva M, Ward RJ, Diemer KM, Yeap SS, Lewiecki EM. Repeating vertebral fracture assessment: 2019 ISCD official position. J Clin Densitom. 2019; 22:484–8.
Article40. Lewiecki EM, Binkley N, Bilezikian JP. Treated osteoporosis is still osteoporosis. J Bone Miner Res. 2019; 34:605–6.
Article41. Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016; 31:16–35.
Article42. Cheung AM, McKenna MJ, van de Laarschot DM, Zillikens MC, Peck V, Srighanthan J, et al. Detection of atypical femur fractures. J Clin Densitom. 2019; 22:506–16.
Article43. Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998; 280:2077–82.
Article