Clin Exp Otorhinolaryngol.
2021 May;14(2):179-184. 10.21053/ceo.2020.00395.
Three-Dimensional Distribution of Cochlear Macrophages in the Lateral Wall of Cleared Cochlea
- Affiliations
-
- 1Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea
- 3Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2515430
- DOI: http://doi.org/10.21053/ceo.2020.00395
Abstract
Objectives
. Resident macrophages are well known to be present in the cochlea, but the exact patterns thereof in spiral ligaments have not been discussed in previous studies. We sought to document the distribution of macrophages in intact cochleae using three-dimensional imaging.
Methods
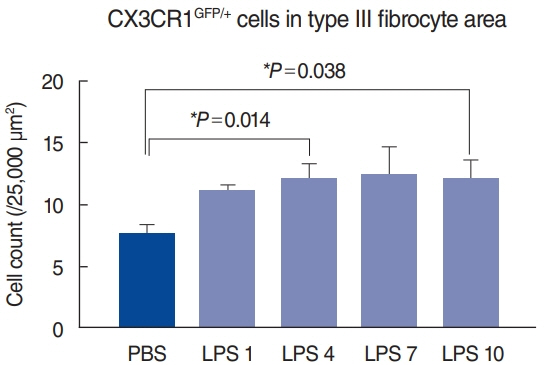
. Cochleae were obtained from C-X3-C motif chemokine receptor 1+/GFP mice, and organ clearing was performed. Three-dimensional images of cleared intact cochleae were reconstructed using two-photon microscopy. The locations of individual macrophages were investigated using 100-μm stacked images to reduce bias. Cochlear inflammation was then induced by lipopolysaccharide (LPS) inoculation into the middle ear through the tympanic membrane. Four days after inoculation, three-dimensional images were obtained.
Results
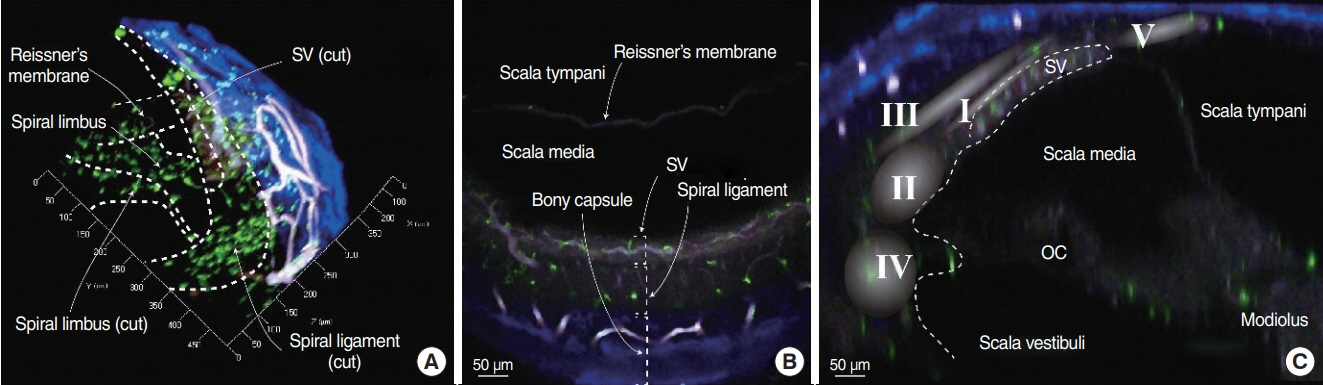
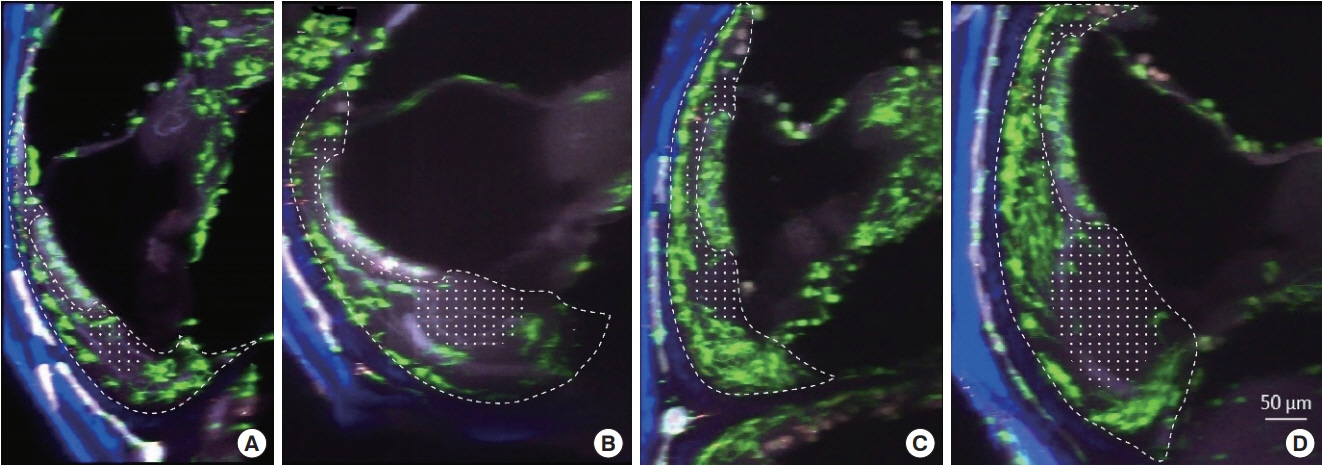
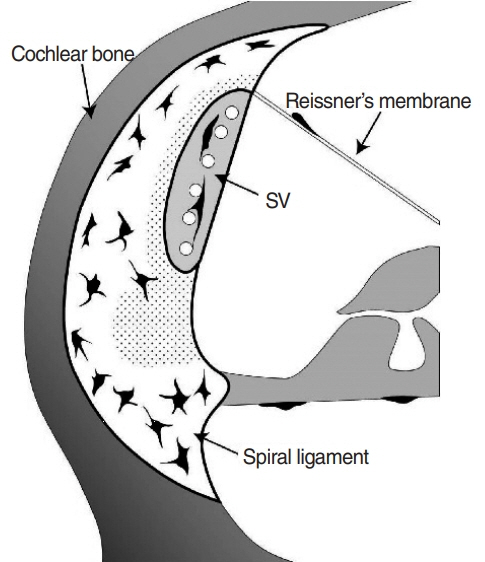
. Macrophages were scarce in areas adjacent to the stria vascularis, particularly the area just beneath it even though many have suspected macrophages to be abundant in this area. This finding remained consistent upon LPS-induced cochlear inflammation, despite a significant increase in the number of macrophages, compared to non-treated cochlea.
Conclusion
. Resident macrophages in spiral ligaments are scarce in areas adjacent to the stria vascularis.
Figure
Cited by 1 articles
-
Inflammatory Monocytes Infiltrate the Spiral Ligament and Migrate to the Basilar Membrane After Noise Exposure
Seung Ho Shin, Jee Eun Yoo, Jinsei Jung, Jae Young Choi, Seong Hoon Bae
Clin Exp Otorhinolaryngol. 2022;15(2):153-159. doi: 10.21053/ceo.2021.00857.
Reference
-
1. Wood MB, Zuo J. The contribution of immune infiltrates to ototoxicity and cochlear hair cell loss. Front Cell Neurosci. 2017; Apr. 11:106.
Article2. Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. 2005; Aug. 489(2):180–94.
Article3. Okano T, Nakagawa T, Kita T, Kada S, Yoshimoto M, Nakahata T, et al. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res. 2008; Jun. 86(8):1758–67.
Article4. Yang W, Vethanayagam RR, Dong Y, Cai Q, Hu BH. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience. 2015; Sep. 303:1–15.
Article5. Tornabene SV, Sato K, Pham L, Billings P, Keithley EM. Immune cell recruitment following acoustic trauma. Hear Res. 2006; Dec. 222(1-2):115–24.
Article6. Kishimoto I, Okano T, Nishimura K, Motohashi T, Omori K. Early development of resident macrophages in the mouse cochlea depends on yolk sac hematopoiesis. Front Neurol. 2019; Oct. 10:1115.
Article7. Liu W, Schrott-Fischer A, Glueckert R, Benav H, Rask-Andersen H. The human “cochlear battery”: claudin-11 barrier and ion transport proteins in the lateral wall of the cochlea. Front Mol Neurosci. 2017; Aug. 10:239.
Article8. Sun GW, Fujii M, Matsunaga T. Functional interaction between mesenchymal stem cells and spiral ligament fibrocytes. J Neurosci Res. 2012; Sep. 90(9):1713–22.
Article9. He W, Yu J, Sun Y, Kong W. Macrophages in noise-exposed cochlea: changes, regulation and the potential role. Aging Dis. 2020; Feb. 11(1):191–9.10. Nakanishi H, Kawashima Y, Kurima K, Chae JJ, Ross AM, Pinto-Patarroyo G, et al. NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc Natl Acad Sci U S A. 2017; Sep. 114(37):E7766–75.
Article11. Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005; Dec. 2(12):932–40.
Article12. Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006; Jun. 50(6):823–39.
Article13. Zhang J, Chen S, Hou Z, Cai J, Dong M, Shi X. Lipopolysaccharideinduced middle ear inflammation disrupts the cochlear intra-strial fluid-blood barrier through down-regulation of tight junction proteins. PLoS One. 2015; Mar. 10(3):e0122572.
Article14. Richter CA, Amin S, Linden J, Dixon J, Dixon MJ, Tucker AS. Defects in middle ear cavitation cause conductive hearing loss in the Tcof1 mutant mouse. Hum Mol Genet. 2010; Apr. 19(8):1551–60.
Article15. Hu BH, Zhang C, Frye MD. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear Res. 2018; May. 362:14–24.
Article16. Miyao M, Firestein GS, Keithley EM. Acoustic trauma augments the cochlear immune response to antigen. Laryngoscope. 2008; Oct. 118(10):1801–8.
Article17. Du X, Choi CH, Chen K, Cheng W, Floyd RA, Kopke RD. Reduced formation of oxidative stress biomarkers and migration of mononuclear phagocytes in the cochleae of chinchilla after antioxidant treatment in acute acoustic trauma. Int J Otolaryngol. 2011; 2011:612690.
Article18. Spicer SS, Schulte BA. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res. 1996; Oct. 100(1-2):80–100.
Article19. Spicer SS, Schulte BA. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear Res. 1991; Nov. 56(1-2):53–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of FGF-R and TGF-R in the Lateral Wall of the Guinea Pig
- Normal Distribution of Nitric Oxide Synthase(NOS) in the Inner Ear of Guinea Pig
- Inflammatory Monocytes Infiltrate the Spiral Ligament and Migrate to the Basilar Membrane After Noise Exposure
- Two Cases of Electrode Kinking in Cochlear Implantation
- Cochlear Implantation Via a Scala Vestibuli in Postmeningitic Ossified Cochlea