Brain Tumor Res Treat.
2021 Apr;9(1):9-15. 10.14791/btrt.2021.9.e7.
The Korean Society for Neuro-Oncology (KSNO) Guideline for Antiepileptic Drug Usage of Brain Tumor: Version 2021.1
- Affiliations
-
- 1Department of Genomic Medicine, Department of Neurology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Division of Neurooncology and Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 4Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 5Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Department of Neurosurgery, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea
- 7Department of Neurosurgery, Catholic Kwandong University, International St. Mary’s Hospital, Incheon, Korea
- 8Department of Neurosurgery, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 9Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 10Department of Neurosurgery, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 11Department of Neurosurgery, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
- 12Department of Neurosurgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 13Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 14Department of Neurosurgery, Yeungnam University Hospital, Yeungnam University College of Medicine, Daegu, Korea
- 15Department of Neurosurgery, DongA University Hospital, Dong-A University College of Medicine, Busan, Korea
- 16Department of Radiation Oncology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 17Department of Radiation Oncology, SMG-SNU Boramae Medical Center, Seoul, Korea
- 18Department of Neurosurgery, Uijeongbu St. Mary’s Hospital, The Catholic University of Korea, Uijeongbu, Korea
- 19Department of Neurosurgery, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- 20Department of Neurosurgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 21Department of Radiation Oncology, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 22Division of Hematology/Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 23Department of Neurosurgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 24Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 25Department of Hospital Pathology, Seoul St. Marry’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 26Department of Cancer Control, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea
- KMID: 2515076
- DOI: http://doi.org/10.14791/btrt.2021.9.e7
Abstract
- Background
To date, there has been no practical guidelines for the prescription of antiepileptic drugs (AEDs) in brain tumor patients in Korea. Thus, the Korean Society for Neuro-Oncology (KSNO), a multidisciplinary academic society, had begun preparing guidelines for AED usage in brain tumors since 2019.
Methods
The Working Group was composed of 27 multidisciplinary medical experts in Korea. References were identified through searches of PubMed, MEDLINE, EMBASE, and Cochrane CENTRAL using specific and sensitive keywords as well as combinations of the keywords.
Results
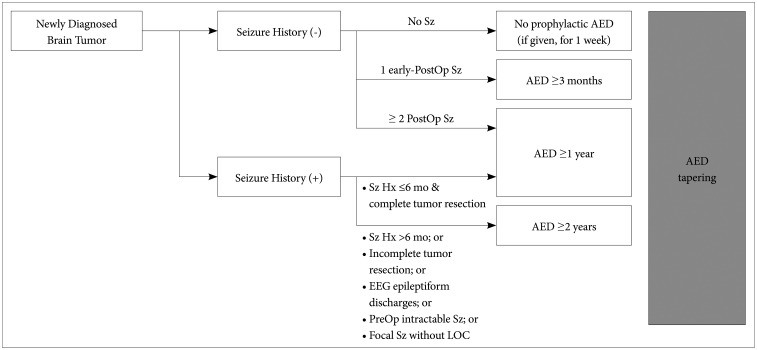
The core contents are as follows. Prophylactic AED administration is not recommended in newly diagnosed brain tumor patients without previous seizure history. When AEDs are administered during peri/postoperative period, it may be tapered off according to the following recommendations. In seizure-naïve patients with no postoperative seizure, it is recommended to stop or reduce AED 1 week after surgery. In seizure-naïve patients with one early postoperative seizure (<1 week after surgery), it is advisable to maintain AED for at least 3 months before tapering. In seizure-naïve patients with ≥2 postoperative seizures or in patients with preoperative seizure history, it is recommended to maintain AEDs for more than 1 year. The possibility of drug interactions should be considered when selecting AEDs in brain tumor patients. Driving can be allowed in brain tumor patients when proven to be seizure-free for more than 1 year.
Conclusion
The KSNO suggests prescribing AEDs in patients with brain tumor based on the current guideline. This guideline will contribute to spreading evidence-based prescription of AEDs in brain tumor patients in Korea.
Figure
Reference
-
1. Ertürk Çetin Ö, İşler C, Uzan M, Özkara Ç. Epilepsy-related brain tumors. Seizure. 2017; 44:93–97. PMID: 28041673.2. Holthausen H, Blümcke I. Epilepsy-associated tumours: what epileptologists should know about neuropathology, terminology, and classification systems. Epileptic Disord. 2016; 18:240–251. PMID: 27506282.3. de Oliveira JA, Santana IA, Caires IQ, et al. Antiepileptic drug prophylaxis in primary brain tumor patients: is current practice in agreement to the consensus? J Neurooncol. 2014; 120:399–403. PMID: 25085213.4. Sayegh ET, Fakurnejad S, Oh T, Bloch O, Parsa AT. Anticonvulsant prophylaxis for brain tumor surgery: determining the current best available evidence. J Neurosurg. 2014; 121:1139–1147. PMID: 25170671.5. Liang S, Fan X, Zhao M, et al. Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma-related epilepsy. Cancer Med. 2019; 8:4527–4535. PMID: 31240876.6. Klimek M, Dammers R. Antiepileptic drug therapy in the perioperative course of neurosurgical patients. Curr Opin Anaesthesiol. 2010; 23:564–567. PMID: 20689411.7. Sughrue ME, Rutkowski MJ, Chang EF, et al. Postoperative seizures following the resection of convexity meningiomas: are prophylactic anticonvulsants indicated? J Neurosurg. 2011; 114:705–709. PMID: 20578801.8. Kim SK, Moon J, Cho JM, et al. A national consensus survey for current practice in brain tumor management I: antiepileptic drug and steroid usage. Brain Tumor Res Treat. 2020; 8:1–10. PMID: 32390348.9. Bauer R, Ortler M, Seiz-Rosenhagen M, Maier R, Anton JV, Unterberger I. Treatment of epileptic seizures in brain tumors: a critical review. Neurosurg Rev. 2014; 37:381–388. discussion 388. PMID: 24760366.10. Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000; 54:1886–1893. PMID: 10822423.11. Siomin V, Angelov L, Li L, Vogelbaum MA. Results of a survey of neurosurgical practice patterns regarding the prophylactic use of anti-epilepsy drugs in patients with brain tumors. J Neurooncol. 2005; 74:211–215. PMID: 16193395.12. Dewan MC, Thompson RC, Kalkanis SN, Barker FG 2nd, Hadjipanayis CG. Prophylactic antiepileptic drug administration following brain tumor resection: results of a recent AANS/CNS Section on Tumors survey. J Neurosurg. 2017; 126:1772–1778. PMID: 27341048.13. Parhi A, Gupta T. Antiepileptic drug usage in neuro-oncology: a practice survey. Int J Neurooncol. 2019; 2:17–23.14. Sirven JI, Wingerchuk DM, Drazkowski JF, Lyons MK, Zimmerman RS. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc. 2004; 79:1489–1494. PMID: 15595331.15. Tremont-Lukats IW, Ratilal BO, Armstrong T, Gilbert MR. Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database Syst Rev. 2008; (2):CD004424. PMID: 18425902.16. Slegers RJ, Blumcke I. Low-grade developmental and epilepsy associated brain tumors: a critical update 2020. Acta Neuropathol Commun. 2020; 8:27. PMID: 32151273.17. Aronica E, Leenstra S, van Veelen CW, et al. Glioneuronal tumors and medically intractable epilepsy: a clinical study with long-term followup of seizure outcome after surgery. Epilepsy Res. 2001; 43:179–191. PMID: 11248530.18. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007; 6:421–430. PMID: 17434097.19. Oberndorfer S, Schmal T, Lahrmann H, Urbanits S, Lindner K, Grisold W. [The frequency of seizures in patients with primary brain tumors or cerebral metastases. An evaluation from the Ludwig Boltzmann Institute of Neuro-Oncology and the Department of Neurology, Kaiser Franz Josef Hospital, Vienna]. Wien Klin Wochenschr. 2002; 114:911–916. PMID: 12528323.20. Baumgarten P, Sarlak M, Baumgarten G, et al. Focused review on seizures caused by meningiomas. Epilepsy Behav. 2018; 88:146–151. PMID: 30269033.21. Lynam LM, Lyons MK, Drazkowski JF, et al. Frequency of seizures in patients with newly diagnosed brain tumors: a retrospective review. Clin Neurol Neurosurg. 2007; 109:634–638. PMID: 17601658.22. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist. 2014; 19:751–759. PMID: 24899645.23. Rudà R, Trevisan E, Soffietti R. Epilepsy and brain tumors. Curr Opin Oncol. 2010; 22:611–620. PMID: 20706121.24. Lee JW, Wen PY, Hurwitz S, et al. Morphological characteristics of brain tumors causing seizures. Arch Neurol. 2010; 67:336–342. PMID: 20212231.25. Lonjaret L, Guyonnet M, Berard E, et al. Postoperative complications after craniotomy for brain tumor surgery. Anaesth Crit Care Pain Med. 2017; 36:213–218. PMID: 27717899.26. Kvam DA, Loftus CM, Copeland B, Quest DO. Seizures during the immediate postoperative period. Neurosurgery. 1983; 12:14–17. PMID: 6828220.27. Boshuisen K, Arzimanoglou A, Cross JH, et al. Timing of antiepileptic drug withdrawal and long-term seizure outcome after paediatric epilepsy surgery (TimeToStop): a retrospective observational study. Lancet Neurol. 2012; 11:784–791. PMID: 22841352.28. Milligan TA, Hurwitz S, Bromfield EB. Efficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgery. Neurology. 2008; 71:665–669. PMID: 18725591.29. Westover MB, Cormier J, Bianchi MT, et al. Revising the “Rule of Three” for inferring seizure freedom. Epilepsia. 2012; 53:368–376. PMID: 22191711.30. Lamberink HJ, Otte WM, Geerts AT, et al. Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. Lancet Neurol. 2017; 16:523–531. PMID: 28483337.31. Kilpatrick CJ. Withdrawal of antiepileptic drugs in seizure-free adults. Aust Prescr. 2004; 27:114–117.32. Koekkoek JA, Kerkhof M, Dirven L, et al. Withdrawal of antiepileptic drugs in glioma patients after long-term seizure freedom: design of a prospective observational study. BMC Neurol. 2014; 14:157. PMID: 25124385.33. Kerkhof M, Koekkoek JAF, Vos MJ, et al. Withdrawal of antiepileptic drugs in patients with low grade and anaplastic glioma after long-term seizure freedom: a prospective observational study. J Neurooncol. 2019; 142:463–470. PMID: 30778733.34. Koekkoek JA, Dirven L, Taphoorn MJ. The withdrawal of antiepileptic drugs in patients with low-grade and anaplastic glioma. Expert Rev Neurother. 2017; 17:193–202. PMID: 27484737.35. Koekkoek JA, Dirven L, Sizoo EM, et al. Symptoms and medication management in the end of life phase of high-grade glioma patients. J Neurooncol. 2014; 120:589–595. PMID: 25151506.36. Bénit CP, Vecht CJ. Seizures and cancer: drug interactions of anticonvulsants with chemotherapeutic agents, tyrosine kinase inhibitors and glucocorticoids. Neurooncol Pract. 2016; 3:245–260. PMID: 31385988.37. Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. 2013; 54:11–27.38. Sepúlveda-Sánchez JM, Conde-Moreno A, Barón M, Pardo J, Reynés G, Belenguer A. Efficacy and tolerability of lacosamide for secondary epileptic seizures in patients with brain tumor: a multicenter, observational retrospective study. Oncol Lett. 2017; 13:4093–4100. PMID: 28599411.39. Rudà R, Pellerino A, Franchino F, et al. Lacosamide in patients with gliomas and uncontrolled seizures: results from an observational study. J Neurooncol. 2018; 136:105–114. PMID: 29030718.40. Maschio M, Zarabla A, Maialetti A, et al. Perampanel in brain tumor-related epilepsy: observational pilot study. Brain Behav. 2020; 10:e01612. PMID: 32285623.41. Coppola A, Zarabla A, Maialetti A, et al. Perampanel confirms to be effective and well-tolerated as an add-on treatment in patients with brain tumor-related epilepsy (PERADET Study). Front Neurol. 2020; 11:592. PMID: 32695064.42. Gefroh-Grimes HA, Gidal BE. Antiepileptic drugs in patients with malignant brain tumor: beyond seizures and pharmacokinetics. Acta Neurol Scand. 2016; 133:4–16. PMID: 25996875.43. Kerrigan S, Grant R. Antiepileptic drugs for treating seizures in adults with brain tumours. Cochrane Database Syst Rev. 2011; (8):CD008586. PMID: 21833969.44. Liguori C, Izzi F, Manfredi N, et al. Efficacy and tolerability of perampanel and levetiracetam as first add-on therapy in patients with epilepsy: a retrospective single center study. Epilepsy Behav. 2018; 80:173–176. PMID: 29414548.45. Li KY, Huang LC, Chang YP, Yang YH. The effects of lacosamide on cognitive function and psychiatric profiles in patients with epilepsy. Epilepsy Behav. 2020; 113:107580. PMID: 33242771.46. Kang JY, Mintzer S. Driving and epilepsy: a review of important issues. Curr Neurol Neurosci Rep. 2016; 16:80. PMID: 27443647.47. Neurological disorders: assessing fitness to drive. Swansea: Driver and Vehicle Licensing Agency;2016. updated Mar 2, 2021. Accessed April 9, 2021. at https://www.gov.uk/guidance/neurological-disorders-assessing-fitness-to-drive.48. Thomas S, Mehta MP, Kuo JS, Ian Robins H, Khuntia D. Current practices of driving restriction implementation for patients with brain tumors. J Neurooncol. 2011; 103:641–647. PMID: 20972603.49. Kang HG, Lee SD, Lee SA, et al. Epilepsy and driving regulation in Korea. J Korean Neurol Assoc. 2018; 36:65–73.50. Cho YW, Kim KT. Driving regulations for epilepsy in South Korea. Epilia. 2020; 2:36–39.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Overview of Practical Guidelines for Gliomas by KSNO, NCCN, and EANO
- A National Consensus Survey for Current Practicein Brain Tumor Management I: Antiepileptic Drug andSteroid Usage
- Yesterdays, Todays, and Tomorrows—Korean Society for Pediatric Neuro-Oncology*
- The Korean Society for Neuro-Oncology (KSNO) Guideline for the Management of Brain Tumor Patients During the Crisis Period: A Consensus Recommendation Using the Delphi Method (Version 2023.1)
- The Korean Society for Neuro-Oncology (KSNO) Guideline for the Management of Brain Tumor Patients During the Crisis Period: A Consensus Survey About Specific Clinical Scenarios (Version 2023.1)