Cancer Res Treat.
2021 Apr;53(2):339-354. 10.4143/crt.2020.790.
The Pattern of Time to Onset and Resolution of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors in Cancer: A Pooled Analysis of 23 Clinical Trials and 8,436 Patients
- Affiliations
-
- 1Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China
- 2Clinical Trials Center, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China
- 3Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, China
- KMID: 2514917
- DOI: http://doi.org/10.4143/crt.2020.790
Abstract
- Purpose
The occurrence pattern of immune-related adverse events (irAEs) induced by immune checkpoint inhibitor (ICI) in cancer treatment remains unclear.
Materials and Methods
Phase II-III clinical trials that evaluated ICI-based treatments in cancer and were published between January 2007 and December 2019 were retrieved from public electronic databases. The pooled median time to onset (PMT-O), resolution (PMT-R), and immune-modulation resolution (PMT-IMR) of irAEs were generated using the metamedian package of R software.
Results
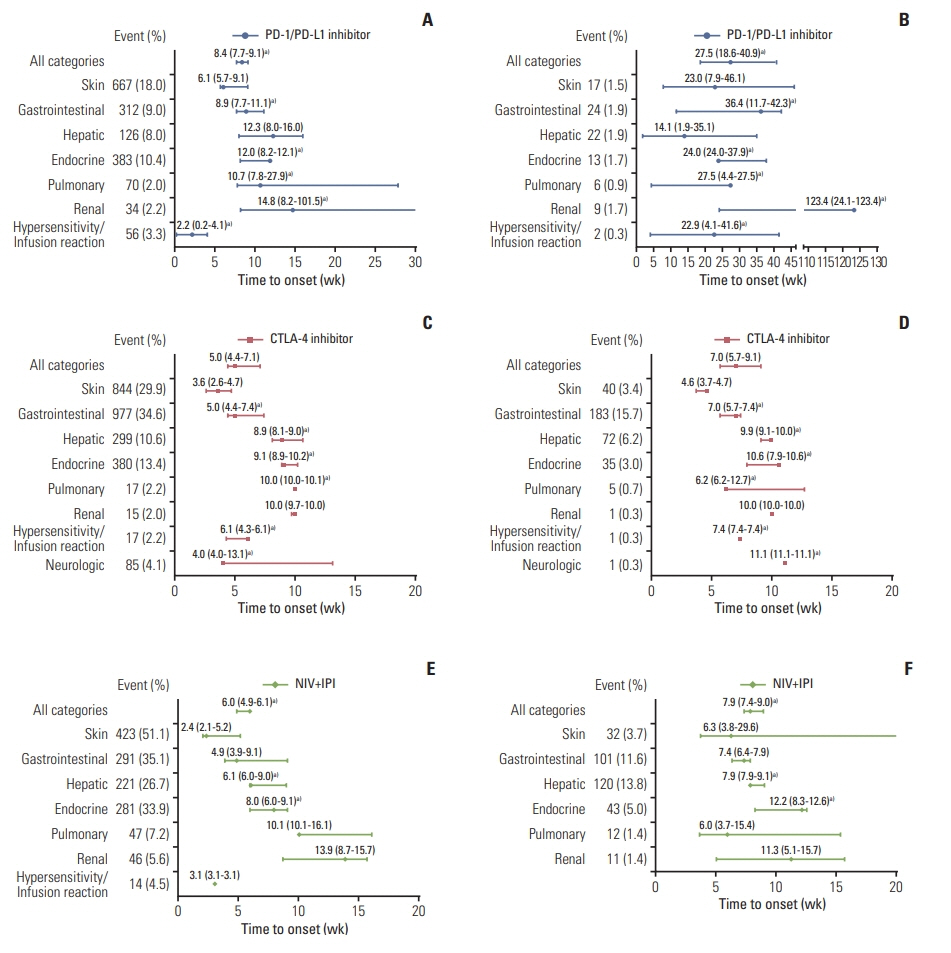
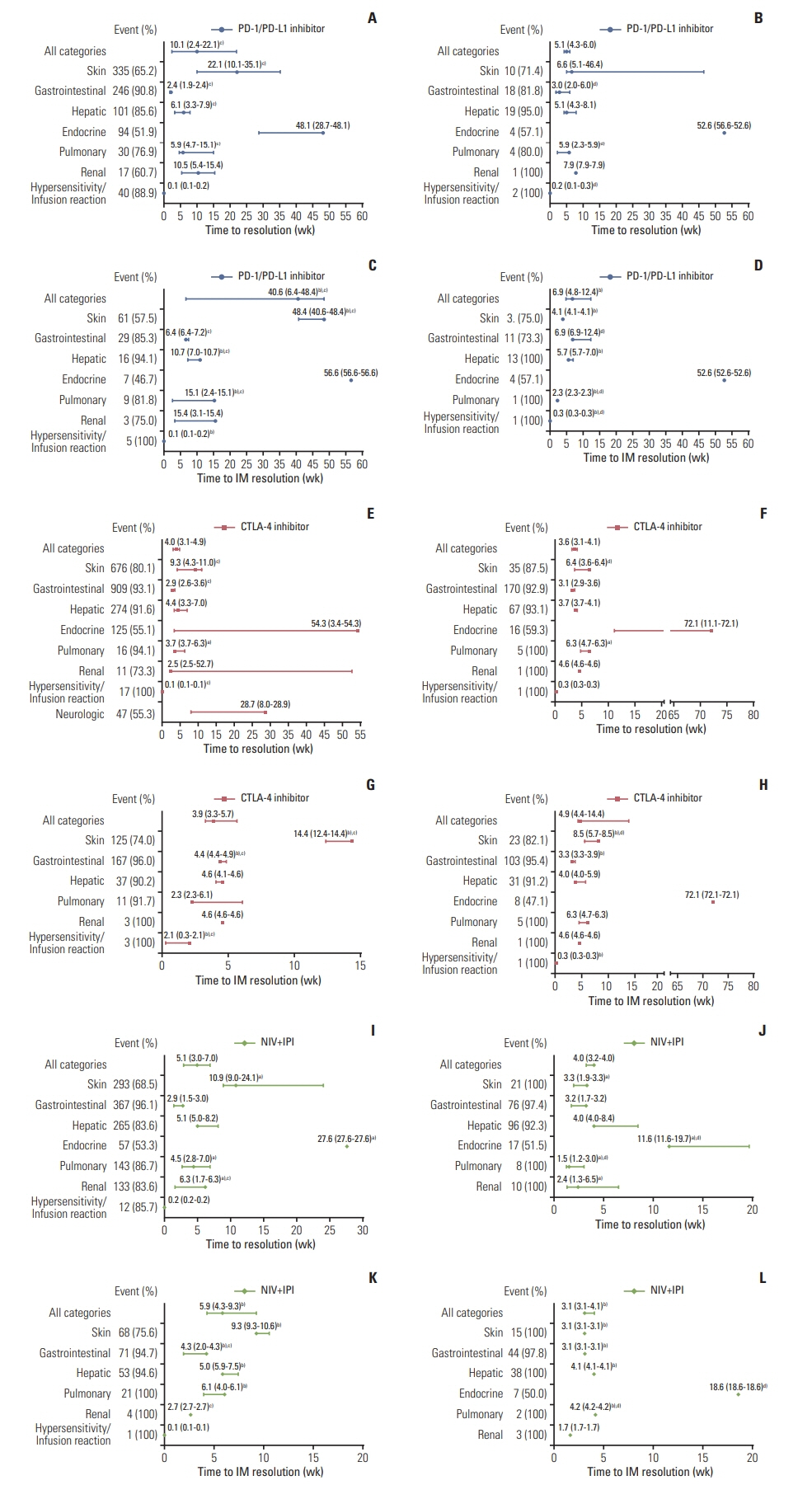
Twenty-two eligible studies involving 23 clinical trials and 8,436 patients were included. The PMT-O of all-grade irAEs ranged from 2.2 to 14.8 weeks, with the longest in renal events. The PMT-O of grade ≥ 3 irAEs was significantly longer than that of all-grade irAEs induced by programmed cell death protein 1 (PD-1) and its ligand 1 (PD-L1) inhibitors (27.5 weeks vs. 8.4 weeks, p < 0.001) and treatment of nivolumab (NIV) plus ipilimumab (IPI) (7.9 weeks vs. 6.0 weeks, p < 0.001). The PMT-R of all-grade irAEs ranged from 0.1 to 54.3 weeks, with the shortest and longest in hypersensitivity/infusion reaction and endocrine events, respectively. The PMT-IMR of grade ≥ 3 irAEs was significantly shorter than that of all-grade irAEs caused by PD-1/PD-L1 blockade (6.9 weeks vs. 40.6 weeks, p=0.002) and NIV+IPI treatment (3.1 weeks vs. 5.9 weeks, p=0.031).
Conclusion
This study revealed the general and specific occurrence pattern of ICI-induced irAEs in pan-cancers, which was deemed to aid the comprehensive understanding, timely detection, and effective management of ICI-induced irAEs.
Figure
Reference
-
References
1. Jain A, Zhang Q, Toh HC. Awakening immunity against cancer: a 2017 primer for clinicians. Chin J Cancer. 2017; 36:67.
Article2. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363:711–23.
Article3. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–35.
Article4. McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015; 33:2013–20.
Article5. Lim SM, Kim SW, Cho BC, Kang JH, Ahn MJ, Kim DW, et al. Real-world experience of nivolumab in non-small cell lung cancer in Korea. Cancer Res Treat. 2020; 52:1112–9.6. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017; 35:785–92.
Article7. Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015; 33:2092–9.
Article8. Johnson DB, Saranga-Perry V, Lavin PJ, Burnette WB, Clark SW, Uskavitch DR, et al. Myasthenia gravis induced by ipilimumab in patients with metastatic melanoma. J Clin Oncol. 2015; 33:e122–4.
Article9. Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014; 16:589–93.
Article10. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018; 391:933.
Article11. Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018; 19:1579–89.
Article12. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, version 1 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2020. [cited 2020 Aug 8]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx .13. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28:iv119–42.
Article14. Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Com- parative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018; 363:k4226.15. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019; 16:563–80.
Article16. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012; 30:2691–7.
Article17. Common Terminology Criteria for Adverse Events version 50 [Internet]. Washington, DC: U.S. Department of Health and Human Services;2017. [cited 2020 Nov 5]. Available from: https://www.eortc.be/services/doc/ctc/CTCAE_v5_Quick_Reference_5x7.pdf .18. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019; 10:ED000142.
Article19. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17:1–12.
Article20. McGrath S, Zhao X, Qin ZZ, Steele R, Benedetti A. One-sample aggregate data meta-analysis of medians. Stat Med. 2019; 38:969–84.
Article21. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS; MDX010-20 Investigators. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013; 119:1675–82.22. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014; 15:700–12.23. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–39.
Article24. Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016; 375:1845–55.
Article25. Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016; 34:3740–8.
Article26. Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017; 18:611–22.
Article27. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017; 377:1824–35.28. Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018; 36:383–90.
Article29. Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017; 35:3449–57.
Article30. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017; 35:3924–33.
Article31. Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018; 36:1428–39.
Article32. Kelly K, Infante JR, Taylor MH, Patel MR, Wong DJ, Iannotti N, et al. Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer. 2018; 124:2010–7.
Article33. Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019; 30:970–6.34. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019; 381:1535–46.
Article35. Geoerger B, Kang HJ, Yalon-Oren M, Marshall LV, Vezina C, Pappo A, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020; 21:121–33.
Article36. Carneiro BA, Konda B, Costa RB, Costa RL, Sagar V, Gursel DB, et al. Nivolumab in metastatic adrenocortical carcinoma: results of a phase 2 trial. J Clin Endocrinol Metab. 2019; 104:6193–200.
Article37. Horinouchi H, Nishio M, Hida T, Nakagawa K, Sakai H, Nogami N, et al. Three-year follow-up results from phase II studies of nivolumab in Japanese patients with previously treated advanced non-small cell lung cancer: pooled analysis of ONO-4538-05 and ONO-4538-06 studies. Cancer Med. 2019; 8:5183–93.
Article38. Lebbe C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol. 2019; 37:867–75.39. Morse MA, Overman MJ, Hartman L, Khoukaz T, Brutcher E, Lenz HJ, et al. Safety of nivolumab plus low-dose ipilimumab in previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Oncologist. 2019; 24:1453–61.
Article40. Sharma P, Sohn J, Shin SJ, Oh DY, Keam B, Lee HJ, et al. Efficacy and tolerability of tremelimumab in locally advanced or metastatic urothelial carcinoma patients who have failed first-line platinum-based chemotherapy. Clin Cancer Res. 2020; 26:61–70.
Article41. Tomita Y, Kondo T, Kimura G, Inoue T, Wakumoto Y, Yao M, et al. Nivolumab plus ipilimumab versus sunitinib in previously untreated advanced renal-cell carcinoma: analysis of Japanese patients in CheckMate 214 with extended follow-up. Jpn J Clin Oncol. 2020; 50:12–9.
Article42. De Velasco G, Je Y, Bosse D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017; 5:312–8.
Article43. Minkis K, Garden BC, Wu S, Pulitzer MP, Lacouture ME. The risk of rash associated with ipilimumab in patients with cancer: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2013; 69:e121–8.
Article44. Tarhini A. Immune-mediated adverse events associated with ipilimumab CTLA-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo). 2013; 2013:857519.
Article45. Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018; 360:k793.
Article46. Almutairi AR, McBride A, Slack M, Erstad BL, Abraham I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: a systematic review and meta-analysis. Front Oncol. 2020; 10:91.
Article47. Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010; 11:155–64.
Article48. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–54.
Article49. Hassel JC, Heinzerling L, Aberle J, Bahr O, Eigentler TK, Grimm MO, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017; 57:36–49.
Article50. Goldinger SM, Stieger P, Meier B, Micaletto S, Contassot E, French LE, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. 2016; 22:4023–9.
Article51. Kahler KC, Hassel JC, Heinzerling L, Loquai C, Mossner R, Ugurel S, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016; 14:662–81.52. Freeman-Keller M, Weber JS. Anti-programmed death receptor 1 immunotherapy in melanoma: rationale, evidence and clinical potential. Ther Adv Med Oncol. 2015; 7:12–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of adverse events in cancer treatment with immune checkpoint inhibitors
- Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
- Immune-related Adverse Events: Overview and Management Strategies for the Use of Immune Checkpoint Inhibitors
- Red Blood Cell Autoantibodies in Patients Treated with Immune Checkpoint Inhibitors
- Immune-Checkpoint Inhibitors in the Era of Precision Medicine: What Radiologists Should Know