Clin Endosc.
2021 Mar;54(2):250-255. 10.5946/ce.2020.091.
Impact of Moderate versus Deep Sedation and Trainee Participation on Adenoma Detection Rate-Analysis of a Veteran Population
- Affiliations
-
- 1Department of Medicine, University of Tennessee Health Science Center, Memphis, TN, USA
- 2Division of Gastroenterology and Hepatology, University of Tennessee Health Science Center, Memphis, TN, USA
- KMID: 2514180
- DOI: http://doi.org/10.5946/ce.2020.091
Abstract
- Background/Aims
The adenoma detection rate (ADR) is used as a quality indicator for screening and surveillance colonoscopy. The study aimed to determine if moderate versus deep sedation affects the outcomes of the ADR and other quality metrics in the veteran population.
Methods
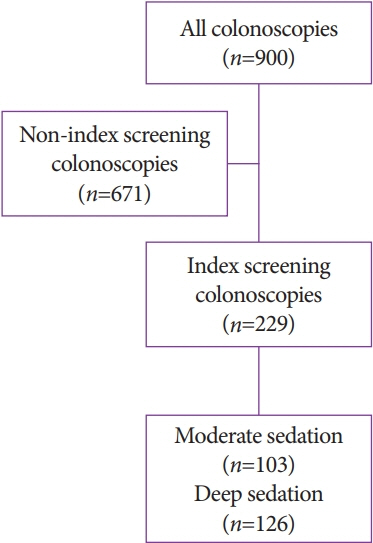
A retrospective review of colonoscopies performed at Memphis Veterans Affairs Medical Center over a one-year period was conducted. A total of 900 colonoscopy reports were reviewed. After exclusion criteria, a total of 229 index, average-risk screening colonoscopies were identified. Data were collected to determine the impact of moderate (benzodiazepine plus opioids) versus deep (propofol) sedation on the ADR, polyp detection rate (PDR), and withdrawal time.
Results
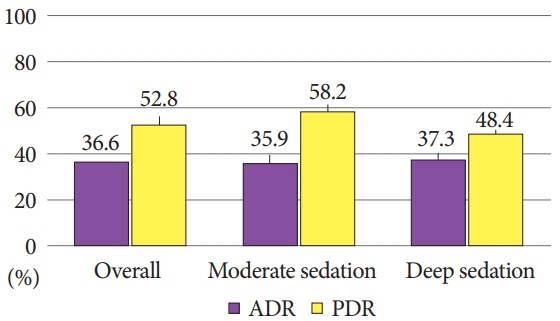
Among 229 screening colonoscopies, 103 (44.9%) used moderate sedation while 126 (55%) were done under deep sedation. The ADR and PDR were not significantly different between moderate versus deep sedation at 35.9% vs. 37.3% (p=0.82) and 58.2% vs. 48.4% (p=0.13), respectively. Similarly, there was no significant difference in withdrawal time between moderate and deep sedation (13.4 min vs. 14 min, p=0.56) during screening colonoscopies.
Conclusions
In veterans undergoing index, average-risk screening colonoscopies, the quality metrics of the ADR, PDR, and withdrawal time are not influenced by deep sedation compared with moderate sedation.
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70:7–30.
Article2. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012; 366:687–696.
Article3. Zullig LL, Williams CD, Fortune-Britt AG. Lung and colorectal cancer treatment and outcomes in the Veterans Affairs health care system. Cancer Manag Res. 2015; 7:19–35.
Article4. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000; 160:3252–3257.5. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010; 25:147–149.
Article6. Park SY, Zhu K, Potter JF, Kolonel LN. Health-related characteristics and dietary intakes of male veterans and non-veterans in the multiethnic cohort study (United States). J Mil Veterans Health. 2011; 19:4–9.7. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016; 315:2564–2575.8. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015; 81:31–53.
Article9. Atia MA, Patel NC, Ratuapli SK, et al. Nonneoplastic polypectomy during screening colonoscopy: the impact on polyp detection rate, adenoma detection rate, and overall cost. Gastrointest Endosc. 2015; 82:370–375.e1.
Article10. Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006; 101:873–885.
Article11. Kilgore TW, Abdinoor AA, Szary NM, et al. Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2011; 73:1240–1245.
Article12. Radaelli F, Paggi S, Hassan C, et al. Split-dose preparation for colonoscopy increases adenoma detection rate: a randomised controlled trial in an organised screening programme. Gut. 2017; 66:270–277.
Article13. Jung Y, Joo YE, Kim HG, et al. Relationship between the endoscopic withdrawal time and adenoma/polyp detection rate in individual colonic segments: a KASID multicenter study. Gastrointest Endosc. 2019; 89:523–530.14. Patel VD, Thompson WK, Lapin BR, Goldstein JL, Yen EF. Screening colonoscopy withdrawal time threshold for adequate proximal serrated polyp detection rate. Dig Dis Sci. 2018; 63:3084–3090.
Article15. Metwally M, Agresti N, Hale WB, et al. Conscious or unconscious: the impact of sedation choice on colon adenoma detection. World J Gastroenterol. 2011; 17:3912–3915.
Article16. Nakshabendi R, Berry AC, Munoz JC, John BK. Choice of sedation and its impact on adenoma detection rate in screening colonoscopies. Ann Gastroenterol. 2016; 29:50–55.17. Radaelli F, Meucci G, Sgroi G, Minoli G. Technical performance of colonoscopy: the key role of sedation/analgesia and other quality indicators. Am J Gastroenterol. 2008; 103:1122–1130.
Article18. Thirumurthi S, Raju GS, Pande M, et al. Does deep sedation with propofol affect adenoma detection rates in average risk screening colonoscopy exams? World J Gastrointest Endosc. 2017; 9:177–182.
Article19. Turse EP, Dailey FE, Bechtold ML. Impact of moderate versus deep sedation on adenoma detection rate in index average-risk screening colonoscopies. Gastrointest Endosc. 2019; 90:502–505.
Article20. Bannert C, Reinhart K, Dunkler D, et al. Sedation in screening colonoscopy: impact on quality indicators and complications. Am J Gastroenterol. 2012; 107:1837–1848.
Article21. Wang A, Hoda KM, Holub JL, Eisen GM. Does level of sedation impact detection of advanced neoplasia? Dig Dis Sci. 2010; 55:2337–2343.
Article22. Enestvedt BK, Eisen GM, Holub J, Lieberman DA. Is the American Society of Anesthesiologists classification useful in risk stratification for endoscopic procedures? Gastrointest Endosc. 2013; 77:464–471.
Article23. El-Halabi MM, Barrett PR, Martinez Mateo M, Fayad NF. Should we measure adenoma detection rate for gastroenterology fellows in training? Gastroenterology Res. 2018; 11:290–294.
Article24. Rogart JN, Siddiqui UD, Jamidar PA, Aslanian HR. Fellow involvement may increase adenoma detection rates during colonoscopy. Am J Gastroenterol. 2008; 103:2841–2846.
Article25. Buchner AM, Shahid MW, Heckman MG, et al. Trainee participation is associated with increased small adenoma detection. Gastrointest Endosc. 2011; 73:1223–1231.
Article26. Peters SL, Hasan AG, Jacobson NB, Austin GL. Level of fellowship training increases adenoma detection rates. Clin Gastroenterol Hepatol. 2010; 8:439–442.
Article27. Bitar H, Zia H, Bashir M, et al. Impact of fellowship training level on colonoscopy quality and efficiency metrics. Gastrointest Endosc. 2018; 88:378–387.
Article28. Qayed E, Shea L, Goebel S, Bostick RM. Association of trainee participation with adenoma and polyp detection rates. World J Gastrointest Endosc. 2017; 9:204–210.29. McCashland T, Brand R, Lyden E, de Garmo P. The time and financial impact of training fellows in endoscopy. CORI research project. Clinical outcomes research initiative. Am J Gastroenterol. 2000; 95:3129–3132.30. Aslanian HR, Shieh FK, Chan FW, et al. Nurse observation during colonoscopy increases polyp detection: a randomized prospective study. Am J Gastroenterol. 2013; 108:166–172.
Article31. Kim TS, Park DI, Lee DY, et al. Endoscopy nurse participation may increase the polyp detection rate by second-year fellows during screening colonoscopies. Gut Liver. 2012; 6:344–348.
Article32. Lee CK, Park DI, Lee SH, et al. Participation by experienced endoscopy nurses increases the detection rate of colon polyps during a screening colonoscopy: a multicenter, prospective, randomized study. Gastrointest Endosc. 2011; 74:1094–1102.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Considerable Variability of Procedural Sedation and Analgesia Practices for Gastrointestinal Endoscopic Procedures in Europe
- Anesthesia and Sedation
- Severe respiratory depression precipitated by unrecognized gastric perforation during endoscopic submucosal dissection under deep sedation: A case report
- Adenoma Detection Rate in Patients Younger Than 50 Years of Age: Relationship of the Adenoma Detection Rate to Interval Cancer
- Effect of Sedation Anesthesia With Intravenous Propofol on Transrectal Ultrasound-Guided Prostate Biopsy Outcomes