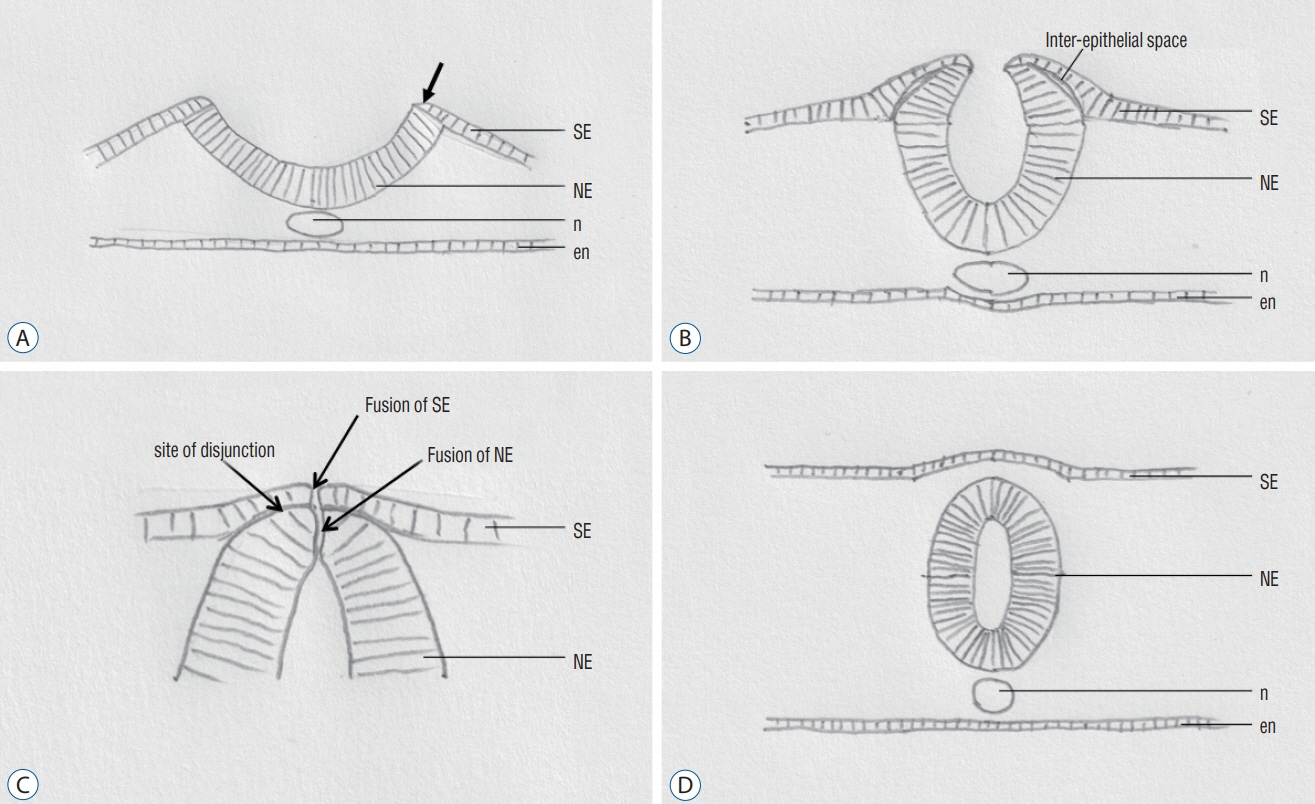
Fig. 1. Normal primary neurulation. Diagrams showing the major steps in closure of the neural groove in an axial level. A : Elevation of the neural folds (arrow). B : Progressive elevation of the neural folds. Delamination at the neuroepithelium – surface epithelium interface. C : Components involved in the final phase in closure of the neural groove. D : Closed neural tube at an axial level. Reused from Wong et al. [43] with permission from Springer Nature. SE : surface epithelium, NE : neuroepithelium, n : notochord, en : endoderm.
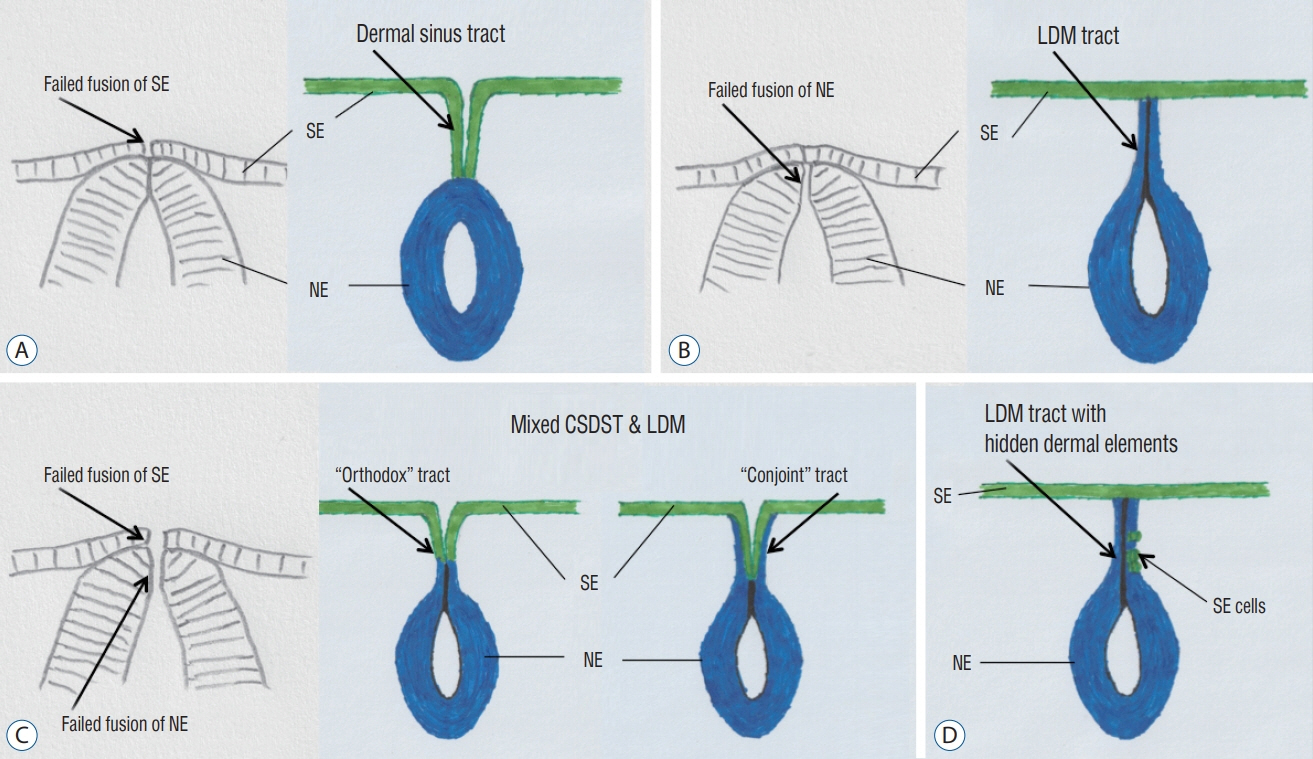
Fig. 2. Proposed embryogenetic mechanisms for different types of focal spinal non-disjunctional disorders. A : Congenital spinal dermal sinus tract (CSDST). B : Limited dorsal myeloschisis (LDM). C : Mixed CSDST and LDM – “orthodox” type and “conjoint” type. D : LDM with hidden dermal elements. SE : surface epithelium, NE : neuroepithelium.
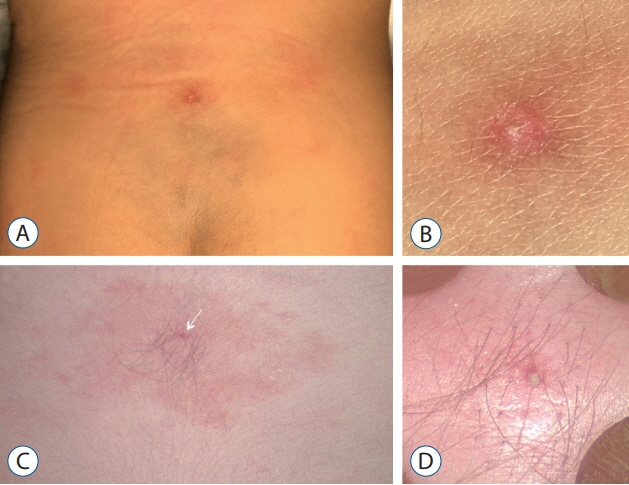
Fig. 3. A : Dermal sinus ostium, appeared as a pin-point area of dry scaling with surrounding red discoloration, but without soft tissue swelling. B : Magnified view of (A). C : Dermal sinus ostium, appeared as a dot of dark discoloration with surrounding hypertrichosis and pigmentation. Keratin material could be seen with light compression. D : Magnified view of (C). Reused from Wong et al. [43] with permission from Springer Nature.
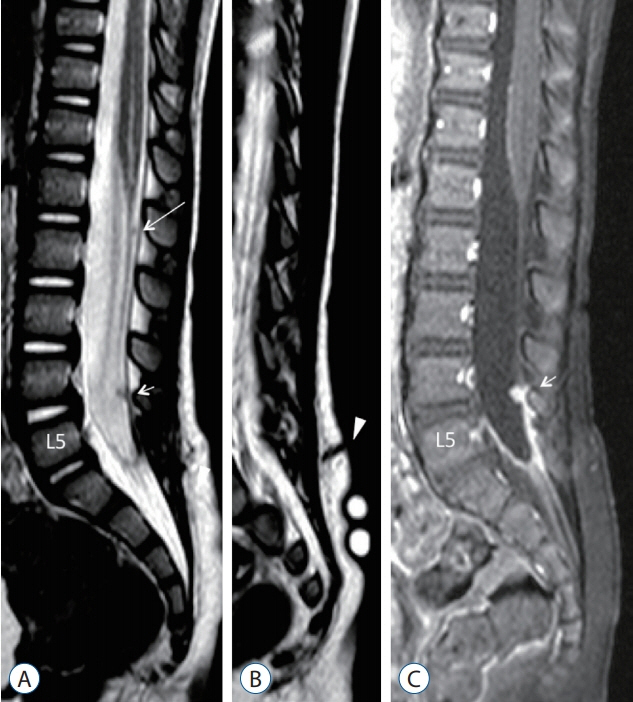
Fig. 4. MRI images of a 22-month-old with a dermal sinus tract, which as confirmed intraoperatively, has the skin ostium at L5 spinous process level (Fig. 3A and B), passes along the caudal aspect of L5 laminae, and terminates on the dorsal surface of the conus. A : Mid-sagittal T2-weighted MRI image showing a tiny T2 hypointense intradural nodule at L1/L2 vertebral level (long arrow), and another slightly larger one at L4 vertebral level (short arrow). The intradural dermal sinus tract is beyond the resolution power of MRI. B : Paramedian sagittal T2-weighted MRI image showing a dermal sinus tract from the skin ostium extending into the subcutaneous fat (arrowhead). C : T1-weighted MRI with gadolinium injection image, corresponding to A, showing that only the L4 lesion (short arrow) and the end of the thecal sac become enhanced due to active inflammation. Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging.

Fig. 5. Right panel: 16 serial T2-weight MRI axial cuts over the lumbosacral region of the patient shown in Fig. 4. Only the L4 nodule (short arrow), the skin ostium and subcutaneous tract (arrowheads), and vaguely the L1/L2 nodule (long arrow) are demonstrable by MRI. Left panel : T2-weighted MRI image with cut lines numbered 1 to 16. Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging, L4 : left lamina of L4 vertebra, L5 : left lamina of L5 vertebra.
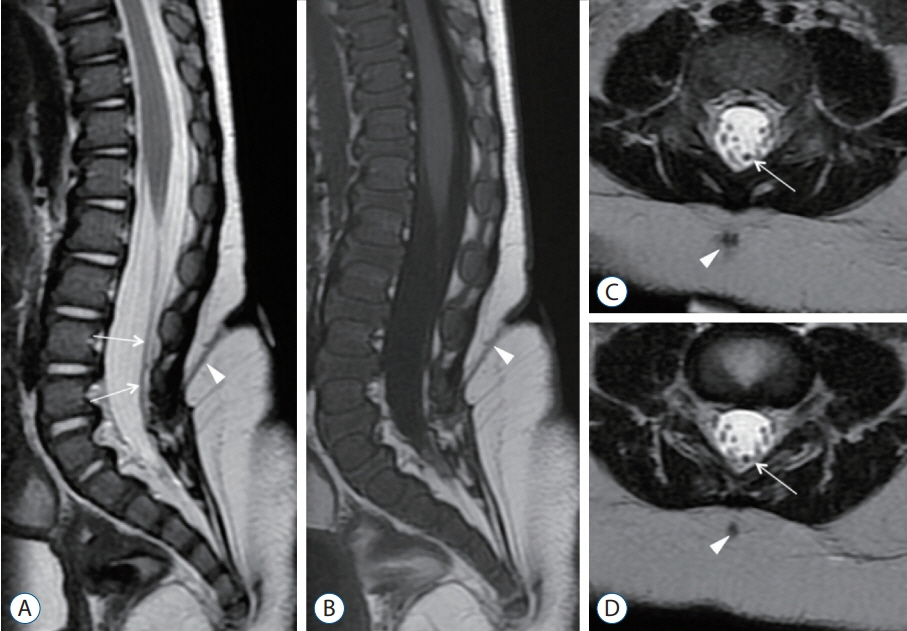
Fig. 6. MRI images of a 20-month-old with a dermal sinus tract. A, C, and D : T2-weighted MRI images. B : T1-weighted MRI image. Although there is marked abnormal signal at the skin level, the skin ostium is tiny (Fig. 3C and D). There is a 2 vertebral levels difference between the skin ostium and where the tract located at the laminar level. The intradural tract (long arrows) appears as a structure that is slightly thicker and more T2 hypointense than normal nerve roots. Arrowheads indicated that the subcutaneous portion of the dermal sinus tract. Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging.
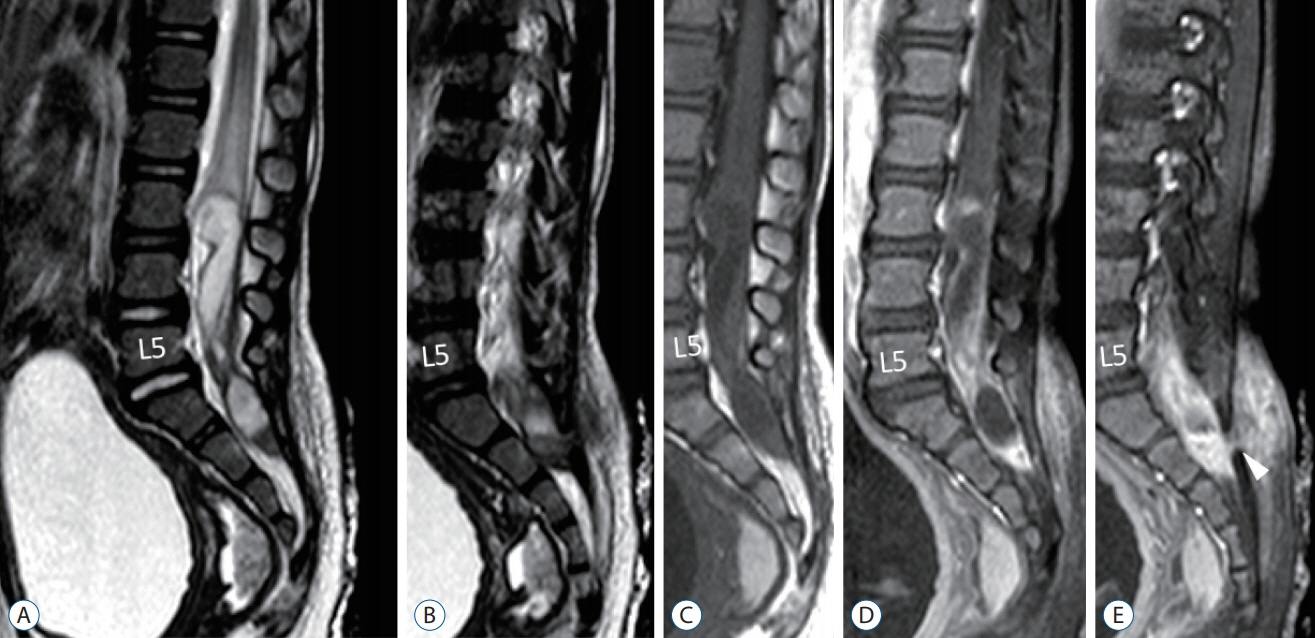
Fig. 7. MRI images of a 25-month-old with a large intradural dermoid cyst. A and B : T2-weighted images. C : T1-weighted image. D and E : T1-weighted with gadolinium injection. The dermoid cyst, spanning 5 vertebral levels, extends from L3 to S2. It is heterogenous in signal intensity, but the main bulk of it is T2-hyperintense, mildly T1-hypointense, and demonstrates periphery gadolinium enhancement. There is also marked gadolinium enhancement in the subcutaneous tissue signifying active inflammation. The intradural dermoid cyst communicates with an outside dermal sinus tract at the caudal aspect of the S1 laminae (white arrowhead in E). Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging.
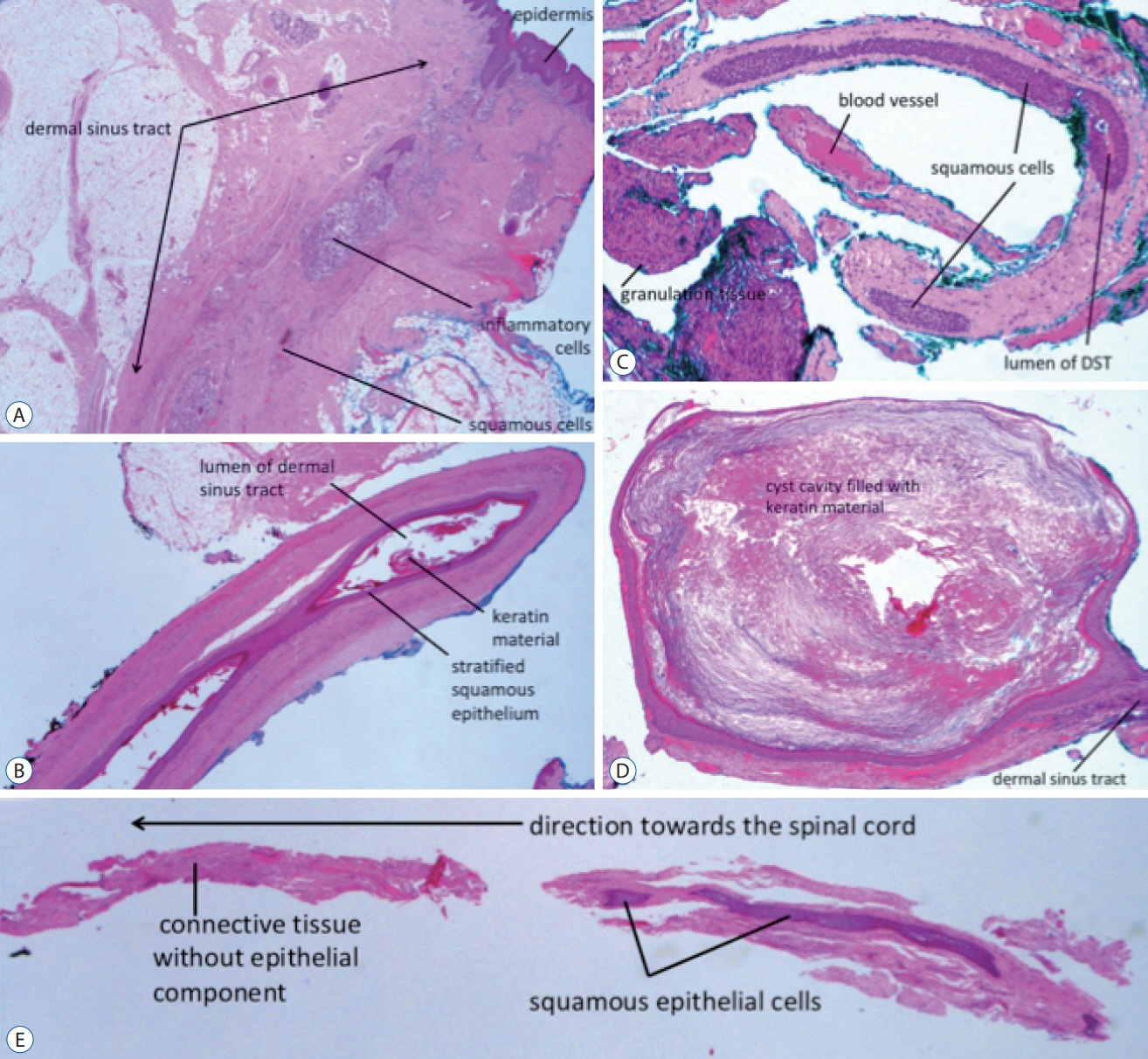
Fig. 8. Histological slides. A : Section through the most superficial portion of a dermal sinus tract. B : The portion of the dermal sinus tract in the intraoperatively identified subcutaneous tissue. C : The intradural portion of a dermal sinus tract (DST). A : The intradural portion of a DST. D : A dermoid cyst formed along a slender dermal sinus tract (the L1/L2 lesion in Fig. 4). E : The deepest end of a dermal sinus tract where a transition from epithelialized tissue to connective tissue totally devoid of it can be seen (haematoxylin and eosin stain). Reused from Wong et al. [43] with permission from Springer Nature.
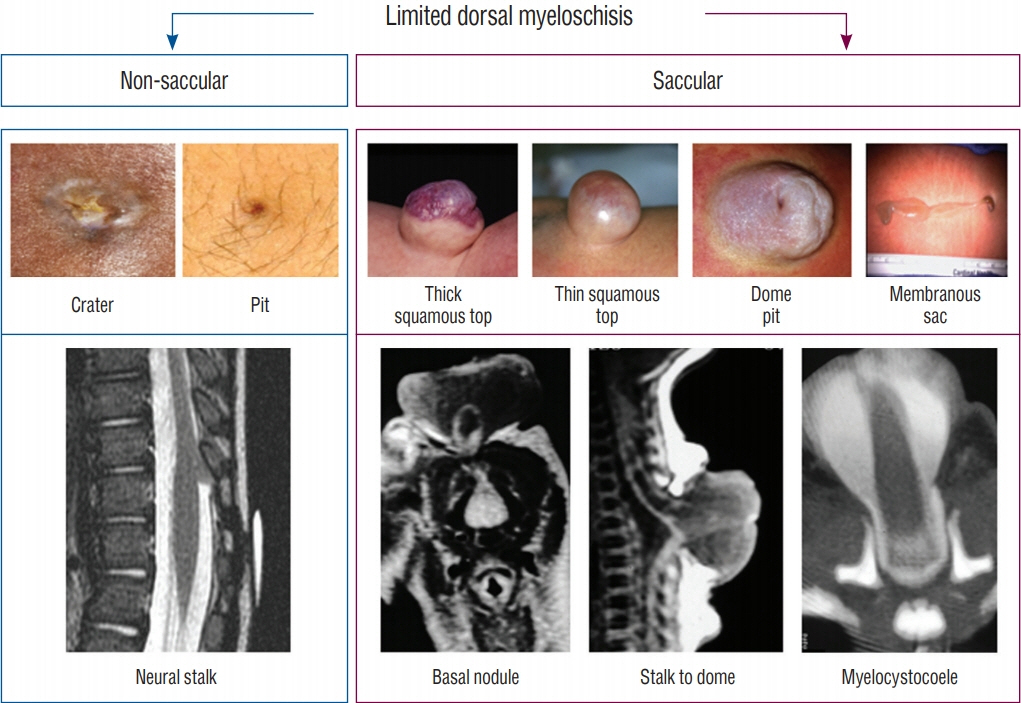
Fig. 9. Classification of limited dorsal myeloschisis (LDM) into non-saccular and saccular types, according to the 2 universal features of LDMs, one external, one internal. The top images depict the various skin signatures of either crater, pit, or the subtypes of sacs. The bottom images feature the internal fibroneural connections between the skin lesion and the spinal cord. Reused from Pang et al. [25] with permission from Springer Nature.
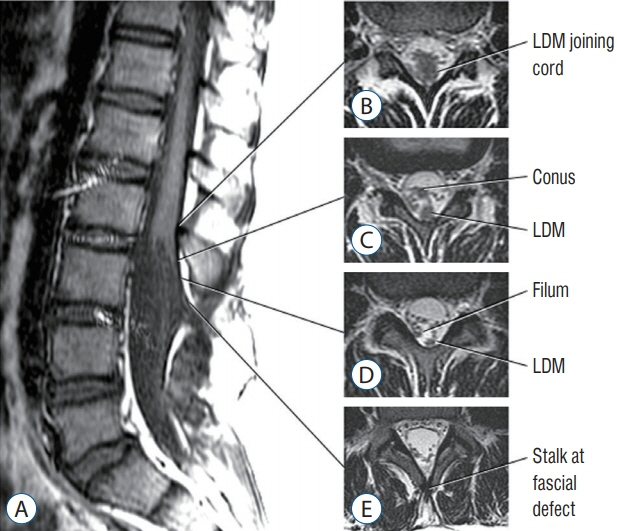
Fig. 10. Lumbar non-saccular (flat) limited dorsal myeloschisis (LDM). A : Sagittal MR showing subcutaneous fibroneural stalk going through laminar defect opposite L3/4, entering dura opposite L3, and joining spinal cord at L2. B : Axial image where LDM stalk joins spinal cord. Note trapezoid shape of the cord-stalk junction. C : Intradural LDM stalk dorsal to the conus (low-lying). D : Intradural LDM stalk dorsal to thickened filum. E : Extradural LDM stalk at laminar defect. Reused from Pang et al. [25] with permission from Springer Nature.
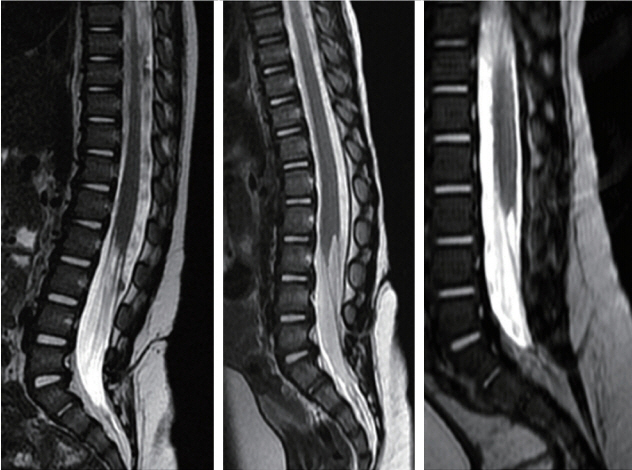
Fig. 11. Three cases of lumbar flat limited dorsal myeloschisis (LDM) with V-shaped course of the LDM stalks. Left : Crater is at L4/5, stalk enters thecal sac at S1, and joins spinal cord at upper margin of L2. Middle : Crater at L3/4, stalk enters dura probably at L5/S1, and joins spinal cord at L2. Right : Crater at L4/5, stalk enters dura around L5/S1, and joins spinal cord at L3. Reused from Pang et al. [25] with permission from Springer Nature.
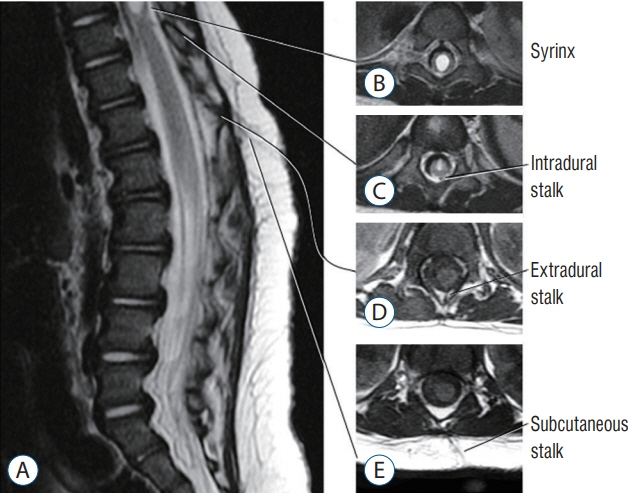
Fig. 12. Upward coursing limited dorsal myeloschisis (LDM) stalk in a thoracic flat LDM. A : Sagittal image shows skin crater at L1, extradural stalk at T12, dural entrance of the stalk above T12, and joining of stalk to spinal cord at T10. B-E : show axial image of the LDM stalk at corresponding points shown in (A). Reused from Pang et al. [25] with permission from Springer Nature.
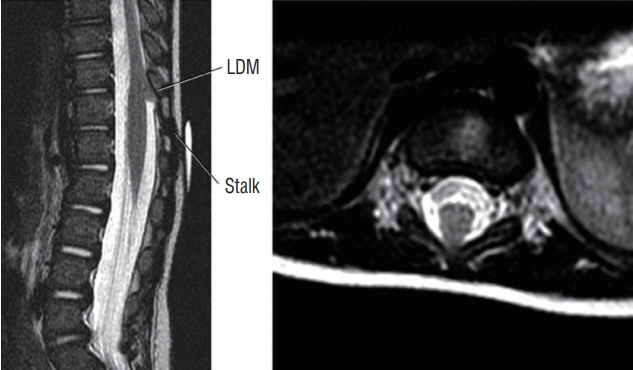
Fig. 13. Thick upward slanting fibroneural stalk in a thoracolumbar (T11/T12) LDM. Reused from Pang et al. [25] with permission from Springer Nature. LDM : limited dorsal myeloschisis.
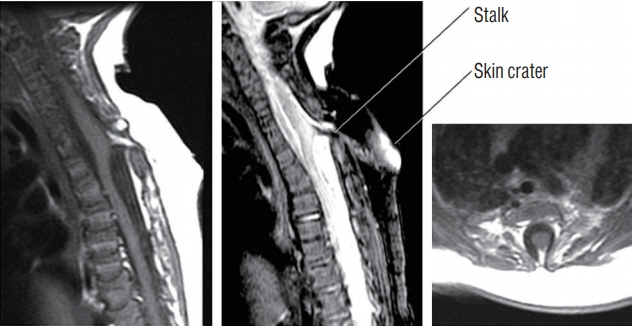
Fig. 14. Upper thoracic crater type limited dorsal myeloschisis (LDM) showing dorsal tenting of both the dural sac and spinal cord at the site of the stalk-cord junction, giving the appearance of taut tethering of the cord. Left : T1 sagittal MRI. Middle : T2 sagittal MRI. Right : Axial MRI. Note skin crater, subcutaneous tract and intradural course of the stalk are well shown on the T2 sagittal image. Also, fat is seen within the stalk. Reused from Pang et al. [25] with permission from Springer Nature. MRI : magnetic resonance imaging.
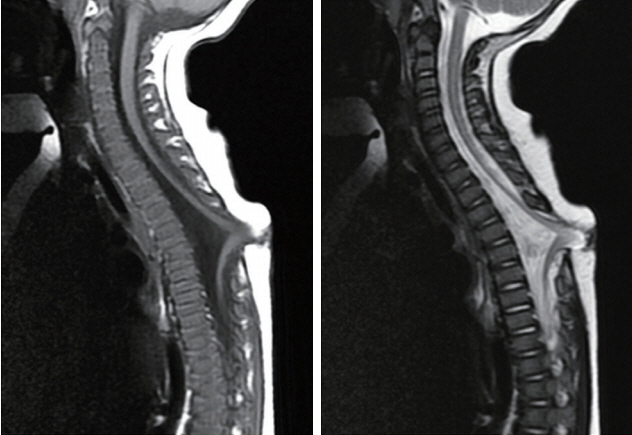
Fig. 15. T5 LDM with non-saccular skin crater showing extreme dural displacement and kinking of the thoracic cord presumably due to “pull” by a short, stout fibroneural stalk. Left : T1 sagittal MRI. Right : T2 sagittal MRI. This child had early neurological deficits. Reused from Pang et al. [25] with permission from Springer Nature. MRI : magnetic resonance imaging.
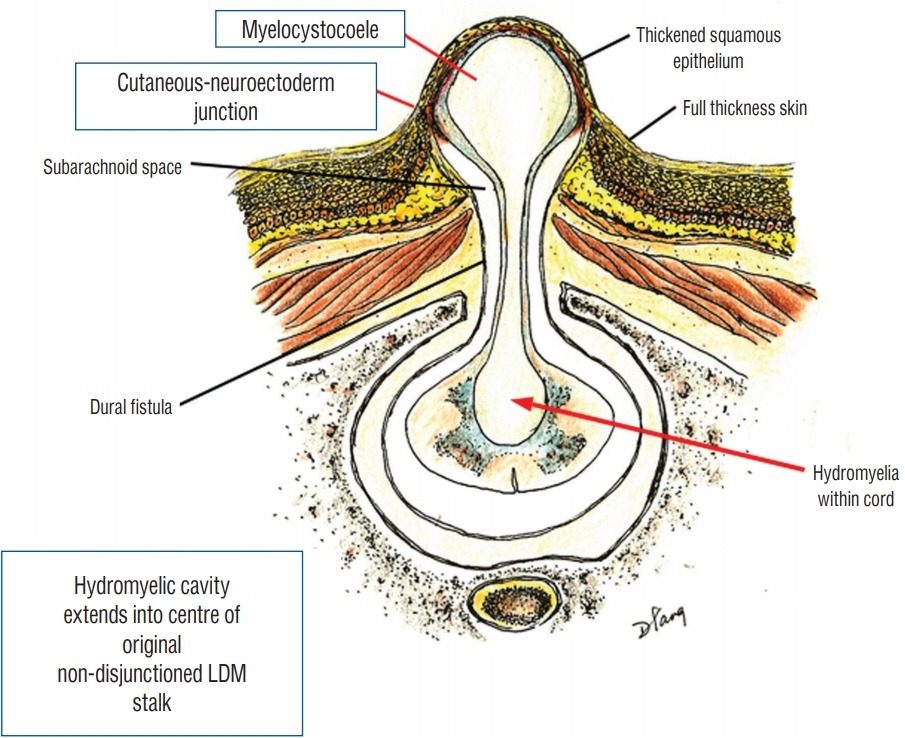
Fig. 16. Formation of saccular limited dorsal myeloschisis (LDM) with segmental myelocystocoele. Fluid from hydromyelic cavity in the underlying spinal cord dissects through the potential tubular space within the original cutaneo neuroectodermal tract and subsequently balloons out into an ependyma-lined myelocystocoele, a sac within an outer sac of distended subarachnoid cerebrospinal fluid. The sac is covered by a full-thickness skin base and a thickened, distinctly different squamous epithelial dome. Reused from Pang et al. [25] with permission from Springer Nature.
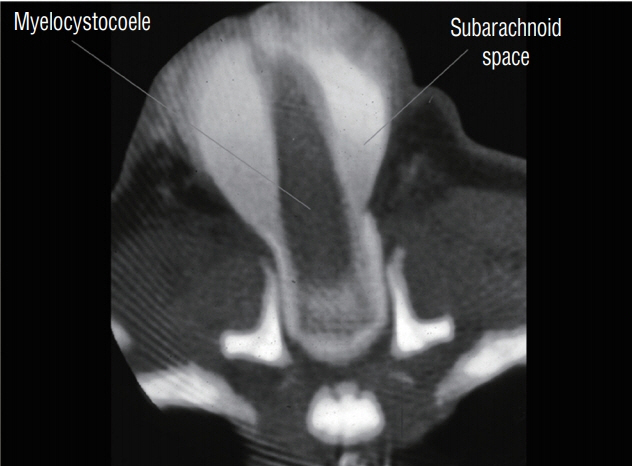
Fig. 17. Computed tomography myelogram of a cervical saccular limited dorsal myeloschisis with segmental myelocystocoele. The myelocystocoele sac does not contain contrast material, which remains in the subarachnoid space. Reused from Pang et al. [25] with permission from Springer Nature.
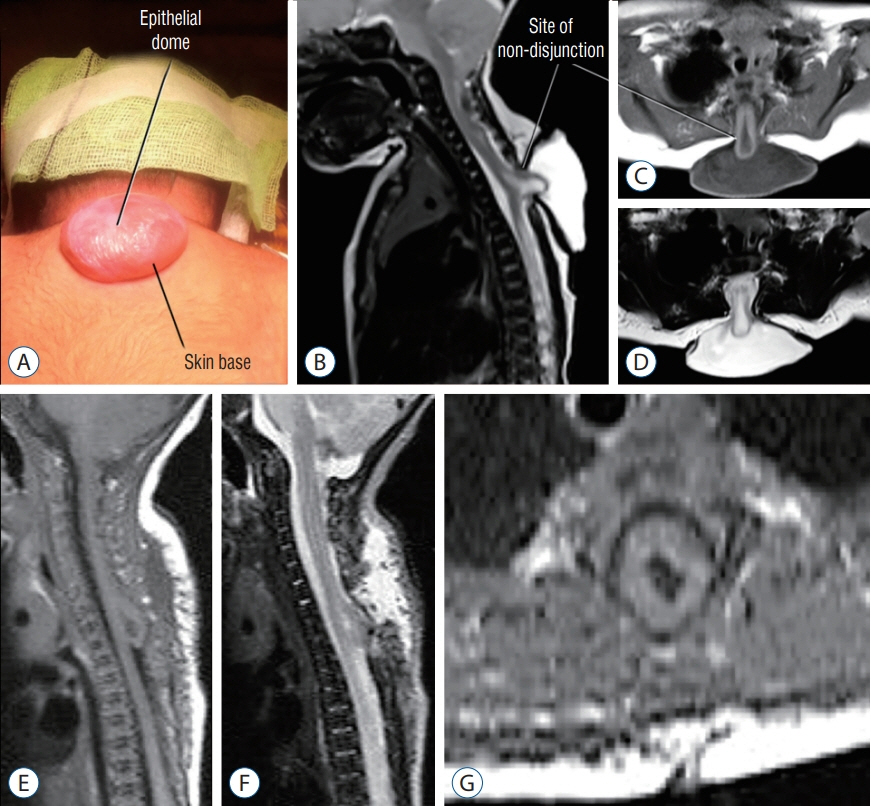
Fig. 18. High thoracic saccular limited dorsal myeloschisis with segmental myelocystocoele. A-D : Pre-operative. A : Photo showing the sac with the epithelial dome. B : Sagittal T2-weighted MRI. C : Axial T1-weighted MRI. D : Axial T2-weighted MRI. E-G : Postoperative MRI images. E : Sagittal T1-weighted MRI. F : Sagittal T2-weighted MRI. G : Axial T1-weighted MRI. MRI : magnetic resonance imaging.
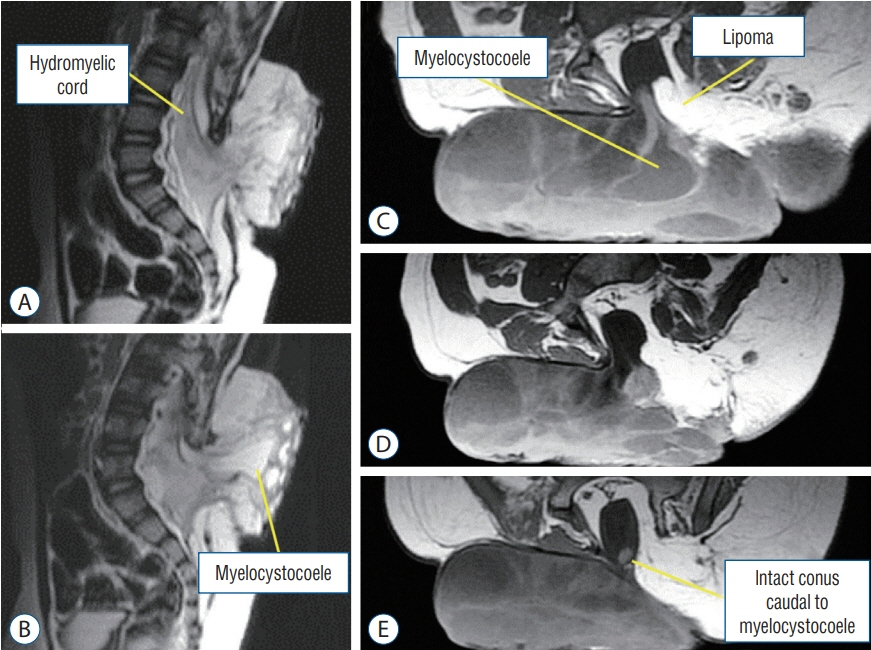
Fig. 19. Lumbar saccular limited dorsal myeloschisis with segmental myelocystocoele and lipoma in a 2.5-year-old boy. A and B : Sagittal T2-weighted MRI. C-E : Axial T1-weighted MRI. MRI : magnetic resonance imaging.
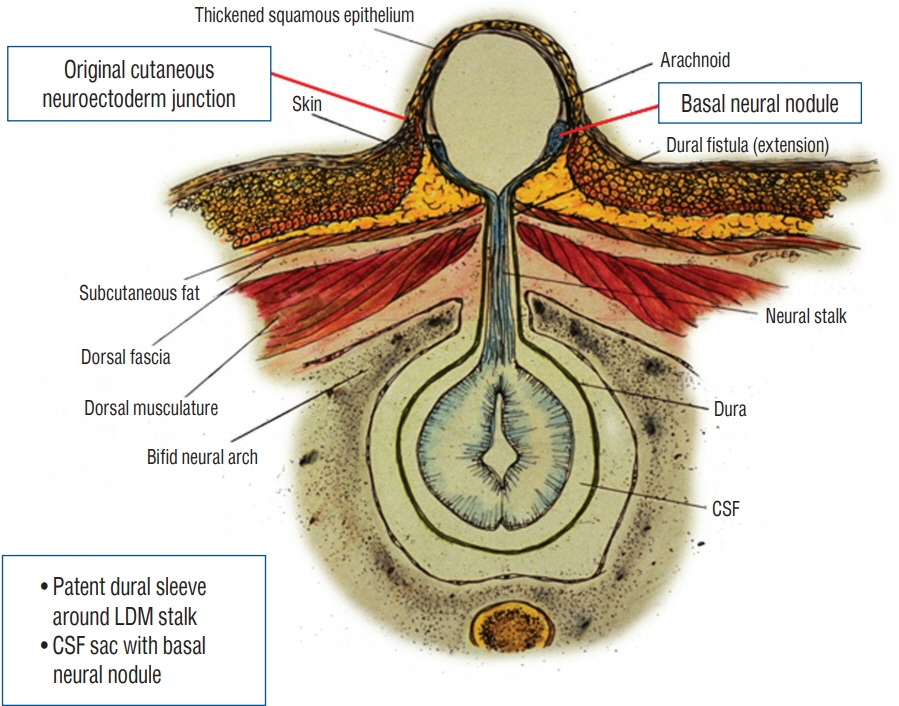
Fig. 20. Formation of a saccular limited dorsal myeloschisis (LDM) with basal neural nodule. In these cases, cerebrospinal fluid (CSF) dissects along the dural fistula ensheathing the fibroneural stalk and balloons out the less well supported midline epithelial layer to give a CSF-filled sac, whose base is skin-covered. The neuroectoderm at the original site of non-disjunction swells to become the basal neural nodule. Reused from Pang et al. [25] with permission from Springer Nature.
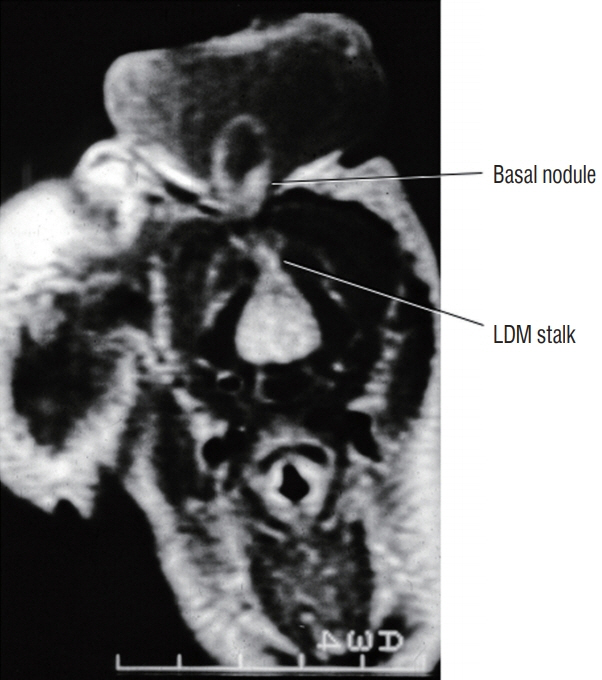
Fig. 21. Cervical saccular limited dorsal myeloschisis (LDM) with basal neural nodule within the base of the cerebrospinal fluid sac at the original non-disjunction site between cutaneous and neural ectoderms. Reused from Pang et al. [25] with permission from Springer Nature.
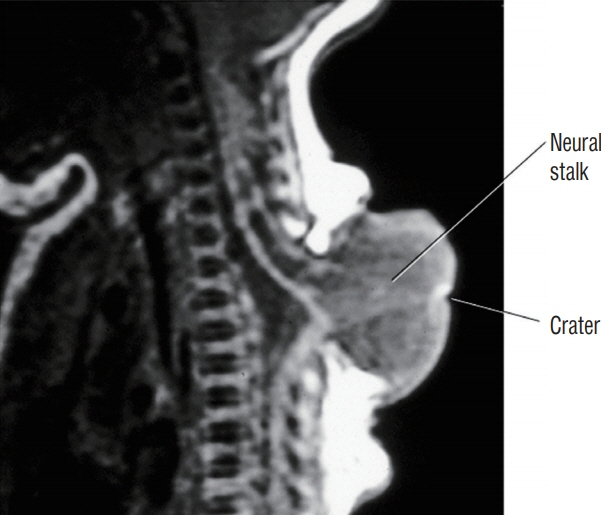
Fig. 22. Thoracic saccular limited dorsal myeloschisis with neural stalk that traverses the cerebrospinal fluid sac and reaches the small skin crater at the top of the cystic dome, presumably the original site of disjunction failure. Reused from Pang et al. [25] with permission from Springer Nature.
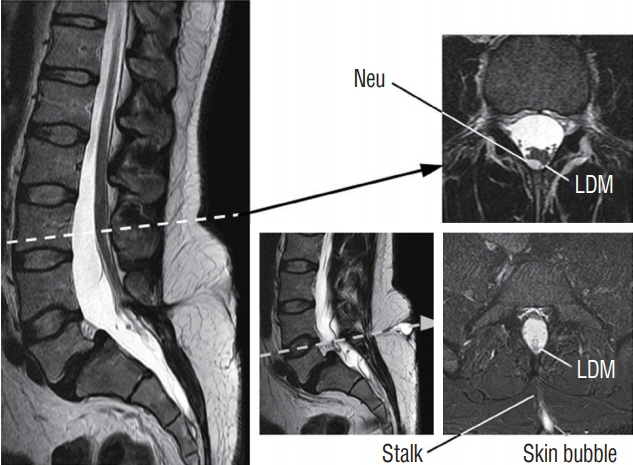
Fig. 23. Lumbar limited dorsal myeloschisis (LDM) showing a cerebrospinal fluid-filled “bubble” topped by squamous epithelium that distends only on straining. Note site of cord-stalk union is with slight dorsal “hump” on the cord outline, and a neurenteric cyst (Neu) right at this site. Reused from Pang et al. [25] with permission from Springer Nature.
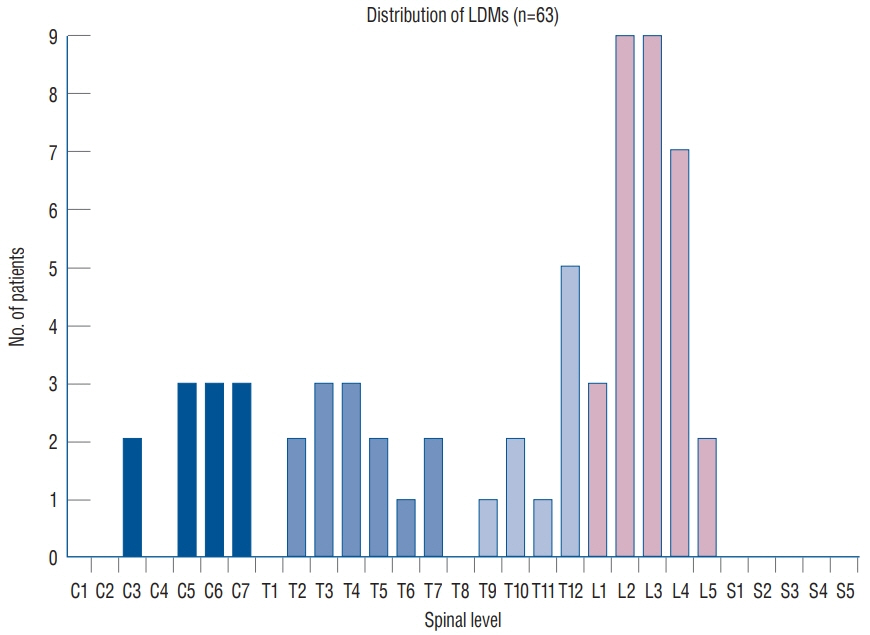
Fig. 24. Distribution of LDMs along the spinal axis. Designation of location is determined by the vertebral level where the fibroneural stalk attaches to the spinal cord. Note the two peaks at L2–L4 and C5–C7, and the absence of sacral lesion. Reused from Pang et al. [25] with permission from Springer Nature. LDM : limited dorsal myeloschisis.
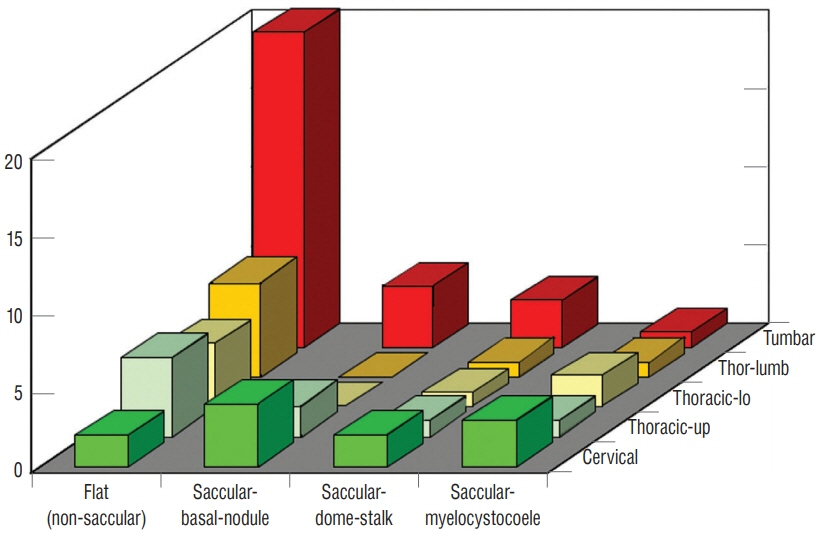
Fig. 25. Distribution of the four types of LDM classified according to external and internal features and assorted by regions of the spinal axis. The four types are flat (non-saccular), saccular with basal neural nodule, saccular with neural stalk reaching the cyst dome, and saccular with segmental myelocystocoele. Note preponderance of the flat LDM in the lumbar and lower thoracic regions. Saccular types are seen in both cervical and lumbar segments. The regions of the vertebral column are coded as : cervical (C1–C7); thoracic-up (T1–T5); thoracic-lo (T5–T11); thor-lumb (T12–L1); lumbar (L1– L5). Reused from Pang et al. [25] with permission from Springer Nature.
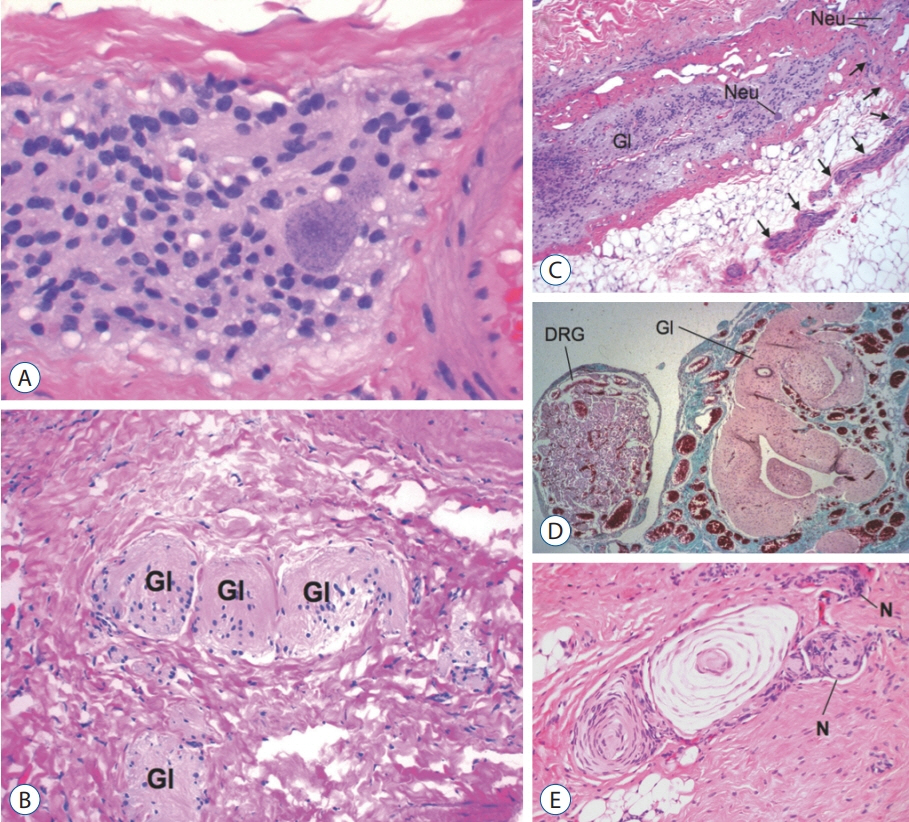
Fig. 26. Histopathology of limited dorsal myeloschisis (LDM) stalk (haematoxylin and eosin stain). A : Core of glial tissue with a large neuron, framed by fibrous tissue. B : Nests of glial tissue (Gl) embedded in a dense fibrous matrix. C : LDM stalk showing a longitudinal glial core (Gl) containing neurons (Neu). A peripheral nerve (arrows) issues forth at right angle to the glial core as from a “real” spinal cord. D : LDM stalk containing glial tissue (Gl) and a large dorsal root ganglion (DRG). E : Peripheral nerves (N) with Pacinian corpuscle within LDM stalk. Pacinian corpuscle in the stalk indicates the nerves involved in LDM formation are indeed sensory nerves likely from the adjacent neural crest. Reused from Pang et al. [25] with permission from Springer Nature.
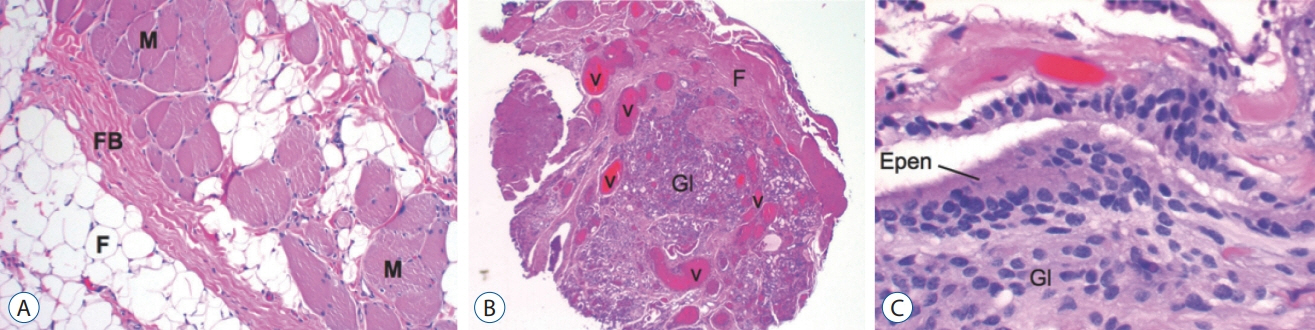
Fig. 27. Histopathology of limited dorsal myeloschisis (LDM) stalk (haematoxylin and eosin stain). A : LDM stalk containing skeletal muscle (M), fat (F), and fibrous band (FB). B : LDM stalk containing prominent blood vessels (V) within a core of glial tissue (Gl), and fibrous bands (F), in the form of a vascular glomus. C : Glioependymal lining of a segmental myelocystocoele in a lumbar saccular LDM. Reused from Pang et al. [25] with permission from Springer Nature. Epen : ependyma, Gl : glial tissue.
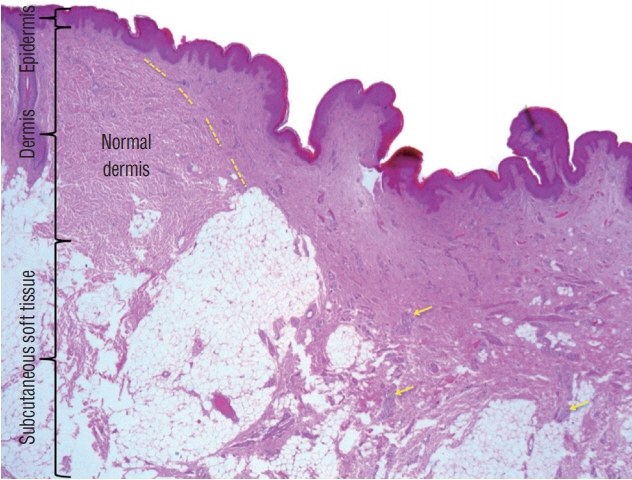
Fig. 28. IHistological slide of a skin “cigarette burn mark” showing increase vascularity, plenty of nerve fibres (yellow arrows), and a different collagen pattern, comparing to the adjacent normal dermis. Yellow dashed line marks the border between normal and abnormal dermis. Gross appearance of the skin lesion is shown in Fig. 29A (haematoxylin and eosin stain). Reused from Wong et al. [43] with permission from Springer Nature.
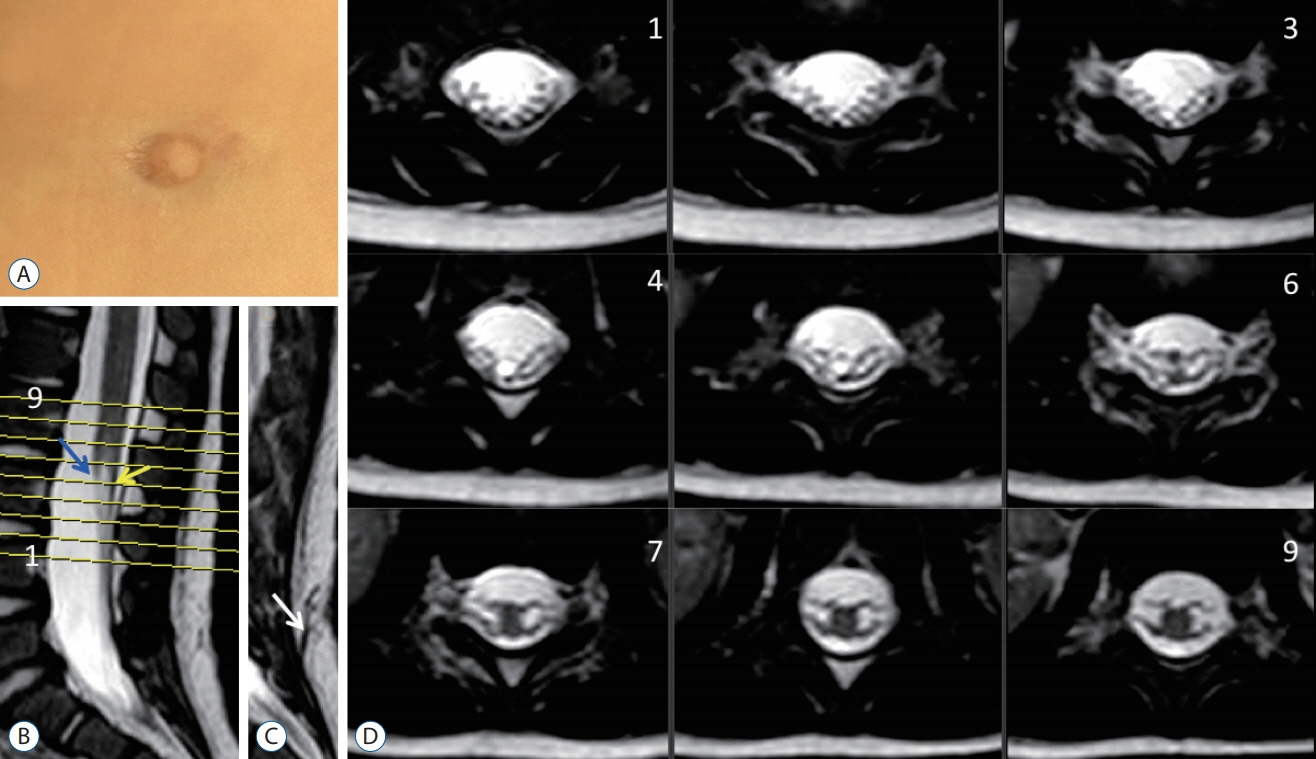
Fig. 29. A case of “clinical and radiolgical” limited dorsal myeloschisis (LDM). A : Cigarette burn mark. B : Mid-sagittal T2 weighted MRI image showing the intradural portion of the LDM stalk (yellow arrow). Blue arrow means the conus. C : Sagittal MRI just next to B showing the subcutaneous portion of the stalk (white arrow). D : Axial T2-weighted MRI images corresponding to the cut lines in (B). Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging.
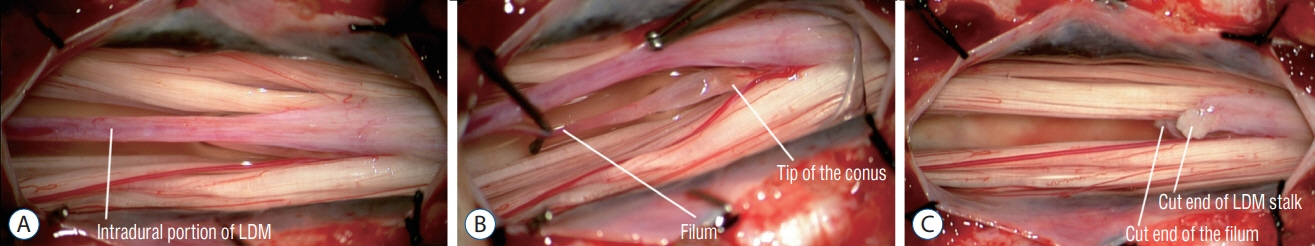
Fig. 30. Intra-operative photographs showing excision of a LDM with a L2L3 laminoplasties. Pre-operative MRI images and the skin lesion are shown in Fig. 29. The skin lesion was traced to the supraspinous ligament of S1 only; the S1 laminae were untouched. This kind of limited exposure thus left a small segment of the LDM stalk in situ. Reused from Wong et al. [43] with permission from Springer Nature. LDM : limited dorsal myeloschisis, MRI : magnetic resonance imaging.
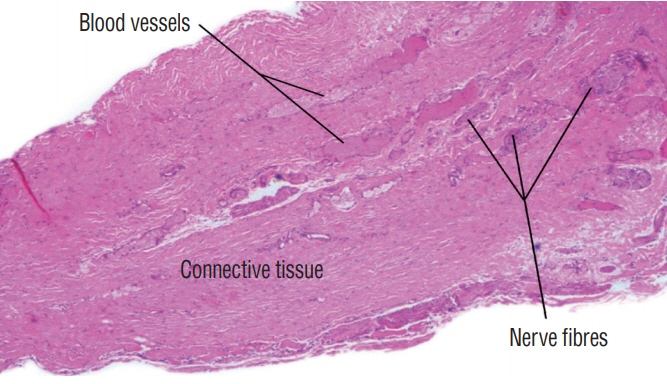
Fig. 31. Histological slide of the intradural portion of a limited dorsal myeloschisis stalk showing the presence of nerve fibres and blood vessels. Intraoperative photograph is shown in Fig. 30 (haematoxylin and eosin stain). Reused from Wong et al. [43] with permission from Springer Nature.
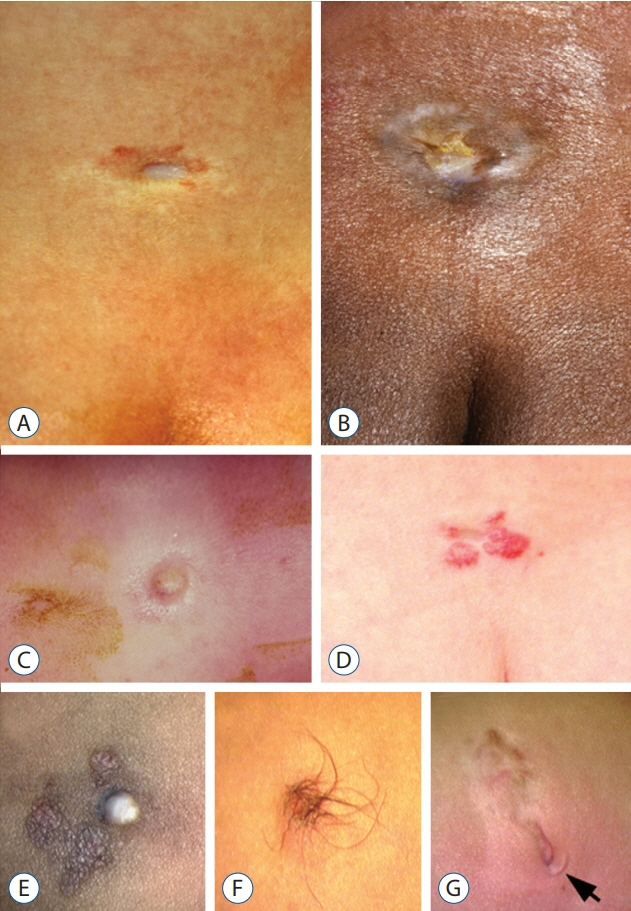
Fig. 32. Flat type skin lesions in limited dorsal myeloschisis. A : Sunken crater of pale squamous epithelium. B : Sunken crater of pale epithelium. C : Squamous epithelial crater with rim of elevated skin borders. D : Crater surrounded by prominent capillary haemangioma with irregular corrugated borders. E : White non-melanotic, epithelial crater with surrounding hyperpigmented skin. F : Crater covered with long hair arising from the rim of surrounding full-thickness skin. G : Crater with surrounding skin overhang (arrow). Reused from Pang et al. [25] with permission from Springer Nature.
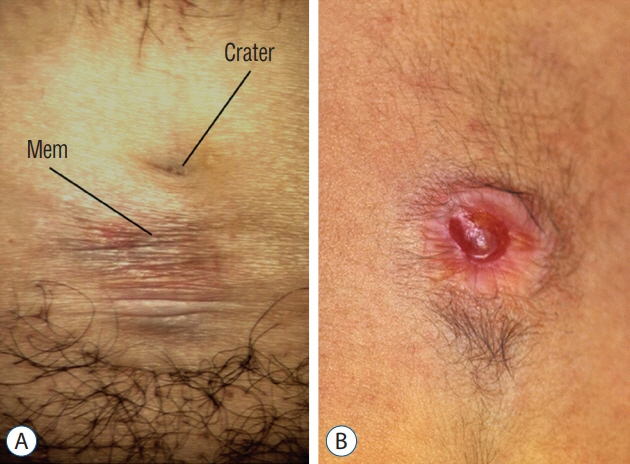
Fig. 33. Flat LDMs with transitional skin lesions: A : Lumbar LDM with a flat epithelial crater and an adjacent area made of stretchable, non-skin epithelium (Mem) that distends into a small CSF-filled bubble when the patient strains. B : Pink epithelial crater slightly distended into a small blister by underlying CSF. Reused from Pang et al. [25] with permission from Springer Nature. LDM : limited dorsal myeloschisis, CSF : cerebrospinal fluid.
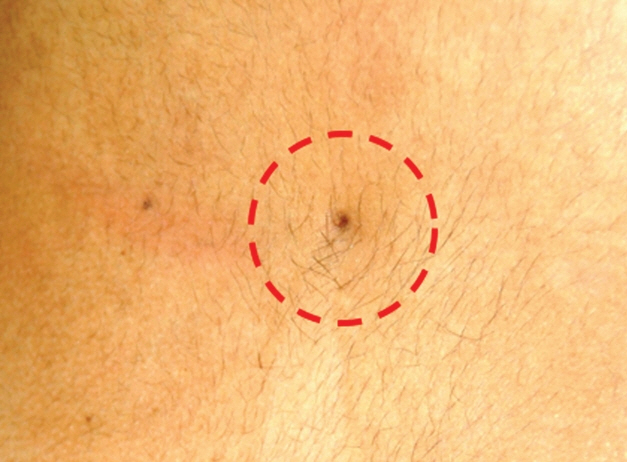
Fig. 34. Subtle pit (within circle) in a flat lumbar limited dorsal myeloschisis with no surrounding exuberance. Reused from Pang et al. [25] with permission from Springer Nature.
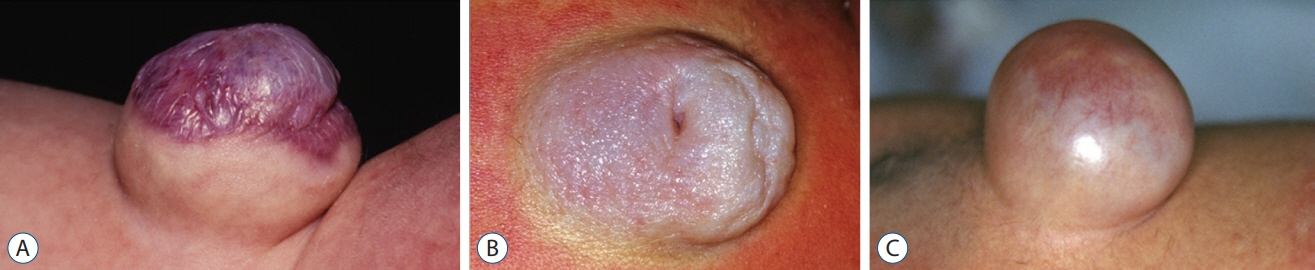
Fig. 35. Saccular skin lesions in limited dorsal myeloschisis (LDM). A : A cervical saccular LDM with full-thickness skin at the base and coarse, thick, corrugated purplish squamous epithelial top. Cervical saccular lesions are usually not turgid. B : An upper thoracic saccular LDM with mostly skin except for a dome crater of squamous epithelium. C : A turgid lumbar saccular LDM with a skin base and a translucent “non-skin” epithelial top. Reused from Pang et al. [25] with permission from Springer Nature.
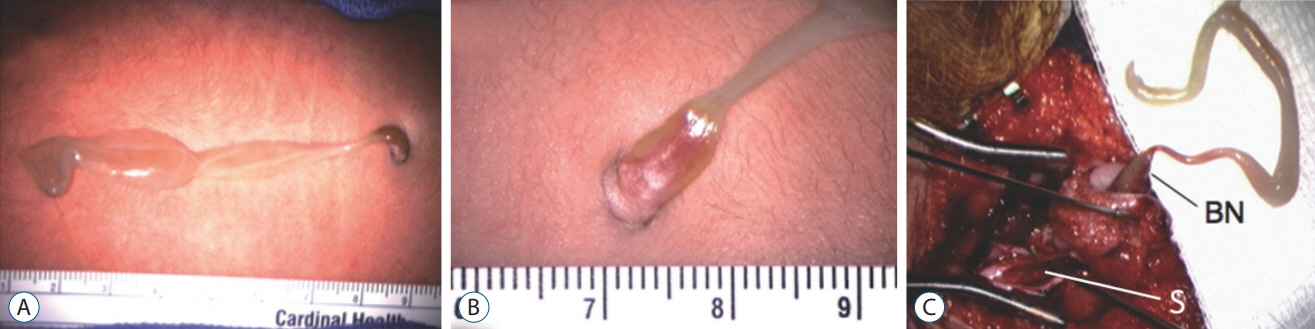
Fig. 36. Lumbar limited dorsal myeloschisis (LDM) with membranous sac. A : Large ruptured sac made of diaphanous membrane. B : Close-up of the base showing a small skin defect through which protrudes a tubular basal neural nodule. C : The entire LDM is exposed at surgery to show basal neural nodules (BN), subcutaneous tract, and intradural stalk (S) attached to the spinal cord. Reused from Pang et al. [25] with permission from Springer Nature.
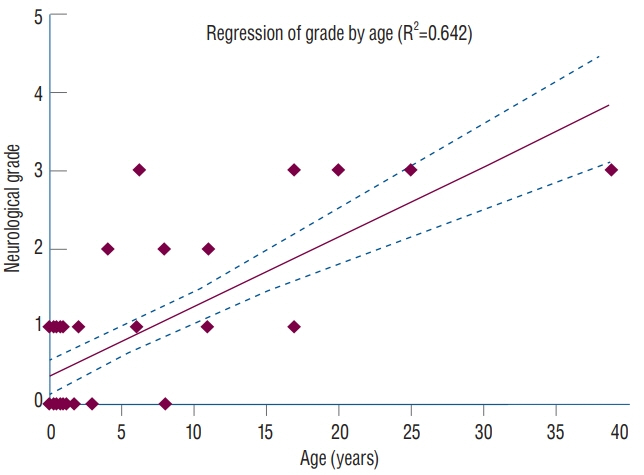
Fig. 37. Linear regression analysis between neurological grade* and patient age shows a logistical tendency for older patients with limited dorsal myeloschisis (LDM) to present with higher grades of neurological deficits (correlative coefficient R2=0.642). Reused from Pang et al. [25] with permission from Springer Nature. *Neurological grading system in LDM : grade 0, no deficits or symptoms; grade 1, mild upper or lower extremity weakness, or pure sensory deficits±pain; grade 2, moderate to severe upper or lower extremity weakness±sensory deficits, or neurogenic bladder without weakness; grade 3, upper or lower extremity weakness+neurogenic bladder.
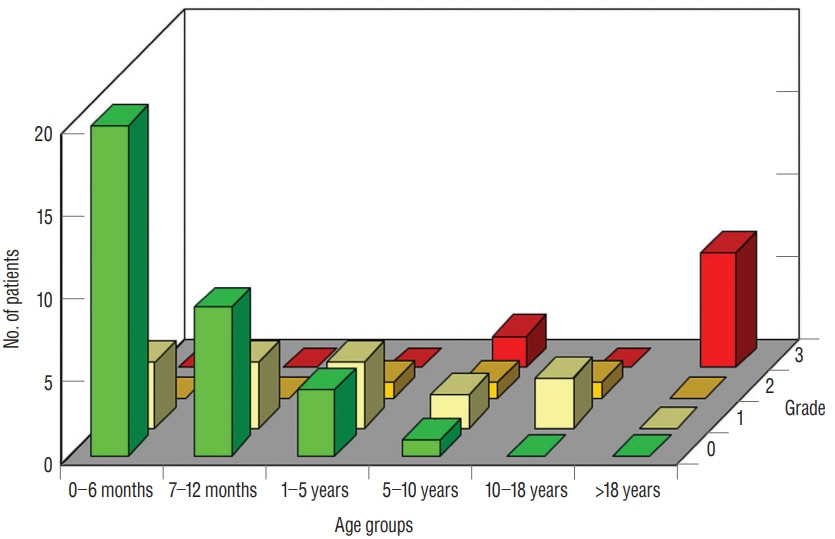
Fig. 38. Clustered bar graphs showing neurological grade assorted by patients’ age-groups (birth to 6 months; 6 to 12 months; 1 to 5 years; 6 to 10 years; 11 to 18 years; and over 18 years) within each neurological grade of 0–3. There is a preponderance of younger children with the better neurological grades and preponderance of older patients with the worse grades. Reused from Pang et al. [25] with permission from Springer Nature.
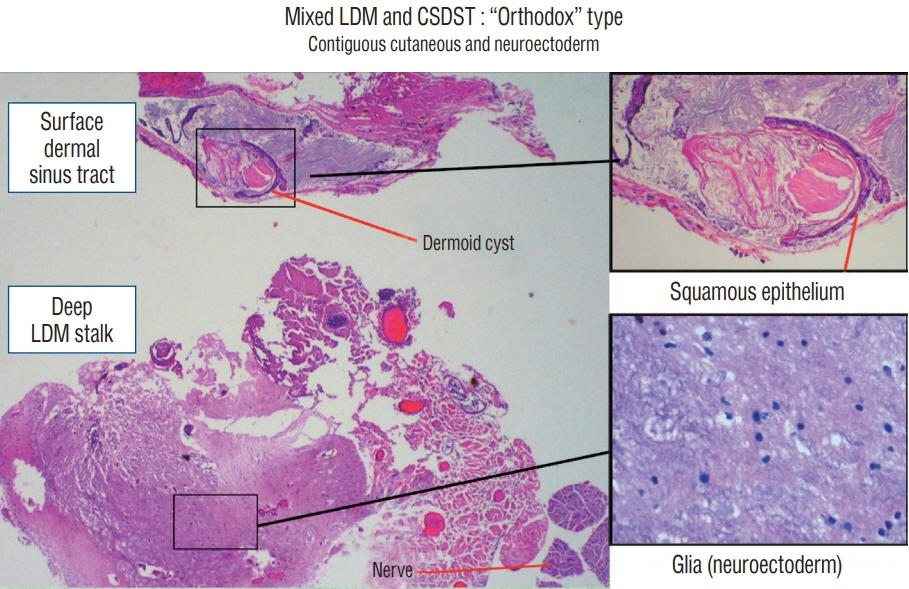
Fig. 39. Histological constituents of a mixed LDM-CSDST stalk of the “orthodox” type. Its superficial portion is of typical dermal tissue lying in tandem with its deeper portion containing mainly glioneuronal tissue. Insets showing both tissues in high power (haematoxylin and eosin stain). LDM : limited dorsal myeloschisis, CSDST : congenital spinal dermal sinus tract.
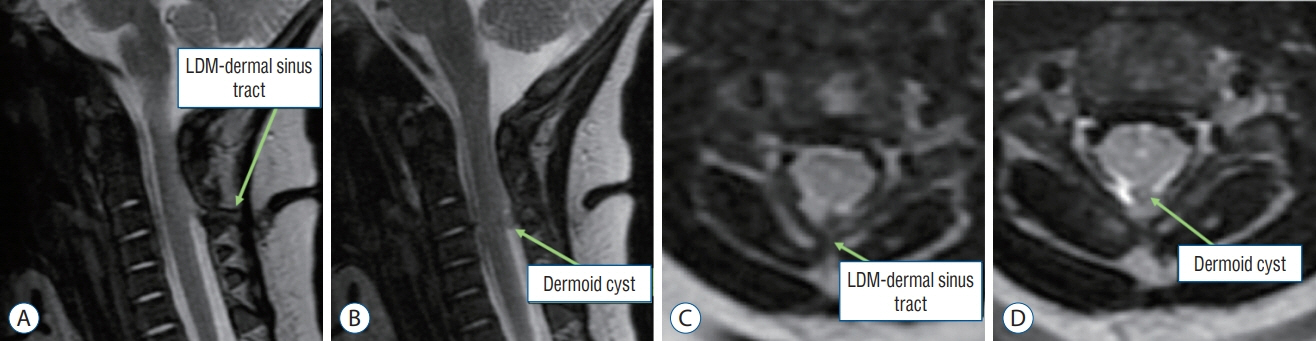
Fig. 40. MRI images of a 3-month-old girl with a mixed LDM and CSDST – “conjoint” type. The diagnosis was made based on intraoperative and histological findings (Figs. 41 and 42). MRI images can only show a tract extending from the skin to the spinal cord, but cannot confirm the components of the tract. A and B : Sagittal T2-weighted MRI images. C and D : Axial T2-weighted MRI images. LDM : limited dorsal myeloschisis, CSDST : congenital spinal dermal sinus tract, MRI : magnetic resonance imaging.
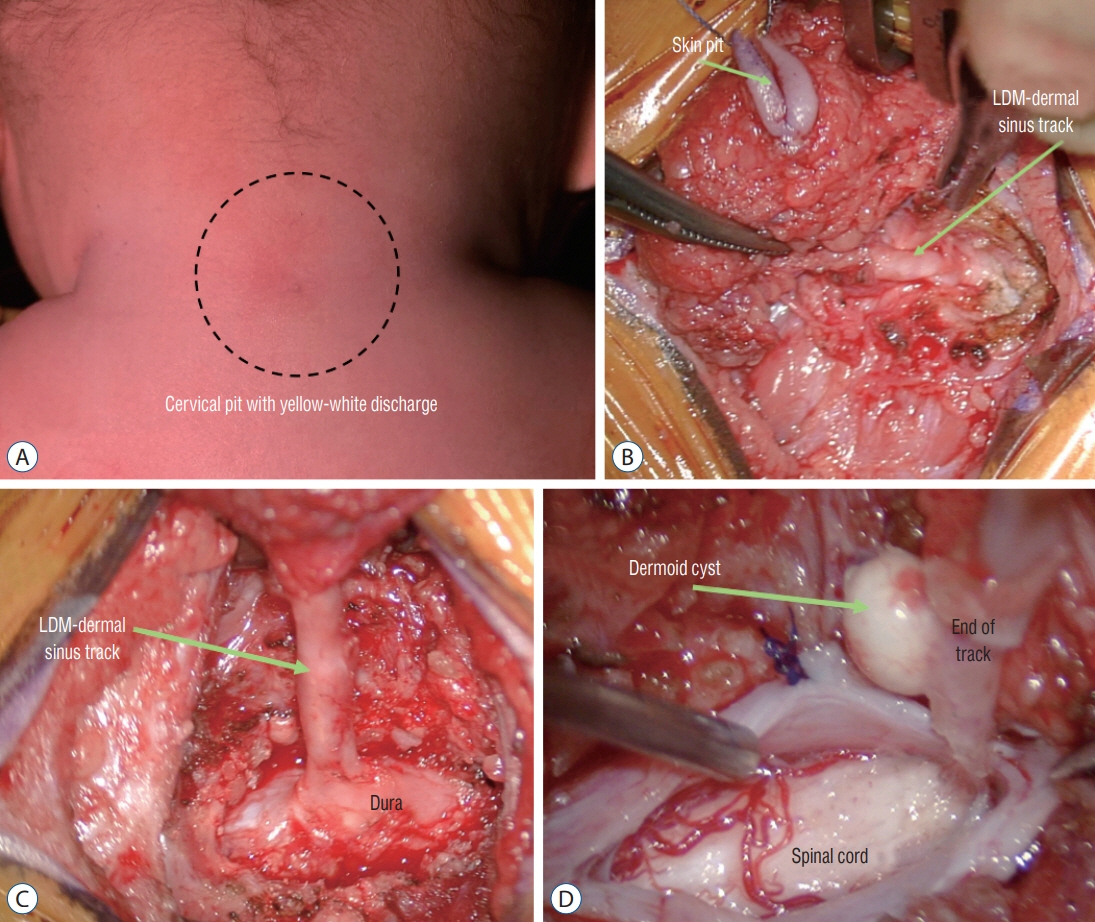
Fig. 41. Intraoperative photos of a patient (Fig. 40) with a mixed LDM and CSDST – “conjoint” type. A : Skin pit. B and C : Dissection of the extra-dural portion of the tract. D : Photo taken at the moment before complete detachment of the tract from the spinal cord. LDM : limited dorsal myeloschisis, CSDST : congenital spinal dermal sinus tract.
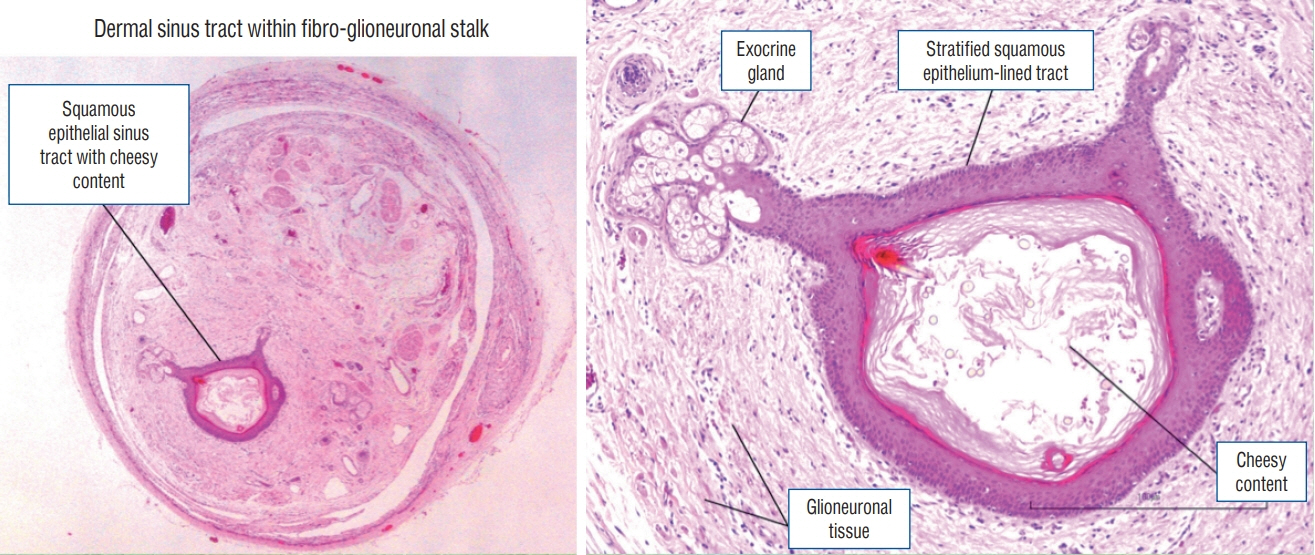
Fig. 42. Histological slides of a patient (Fig. 40) with a mixed LDM and CSDST – “conjoint” type showing the presence of a dermal sinus tract within a fibro-glioneuronal stalk (haematoxylin and eosin stain). LDM : limited dorsal myeloschisis, CSDST : congenital spinal dermal sinus tract.
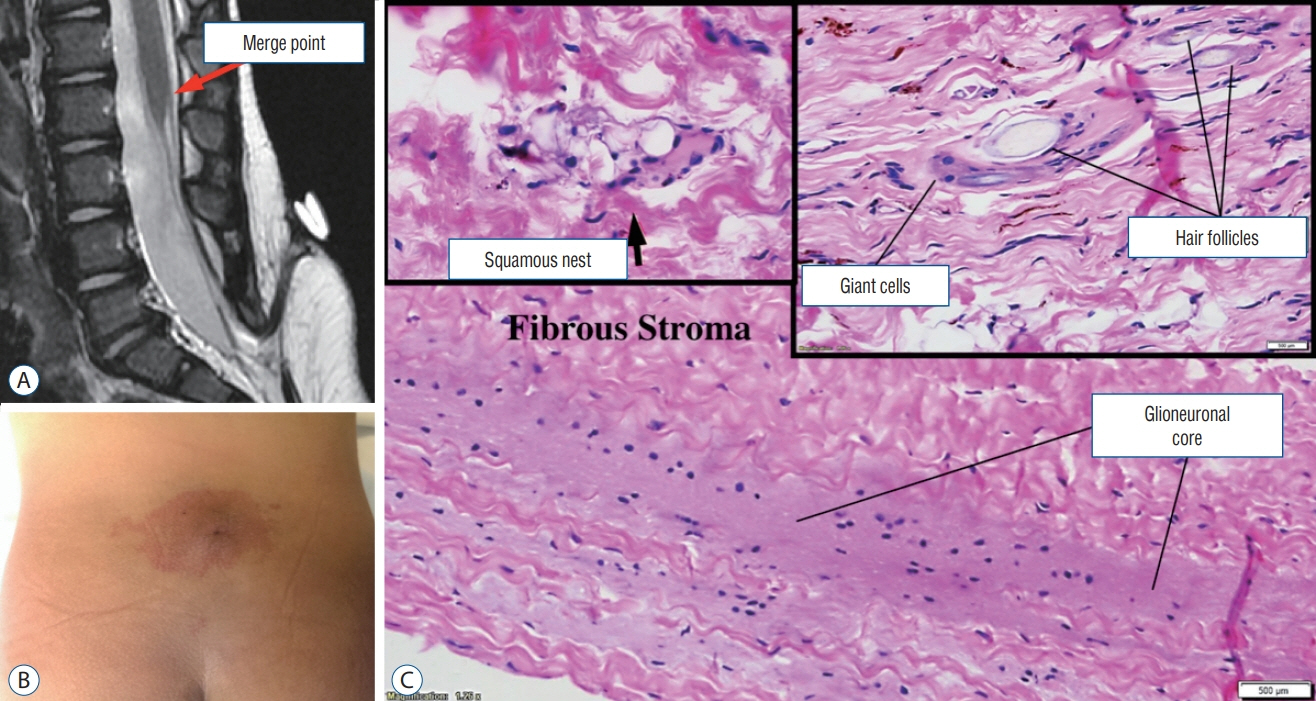
Fig. 43. A 15-month-old with a LDM with hidden dermal elements. A : Sagittal T2-weighted MRI showing the appearance of a classic lumbar LDM. B : Photo showing a crater and surrounding haemangioma. C : Histological slide showing a stalk with glioneuronal core and derivatives of squamous epithelium (haematoxylin and eosin stain). LDM : limited dorsal myeloschisis, MRI : magnetic resonance imaging.
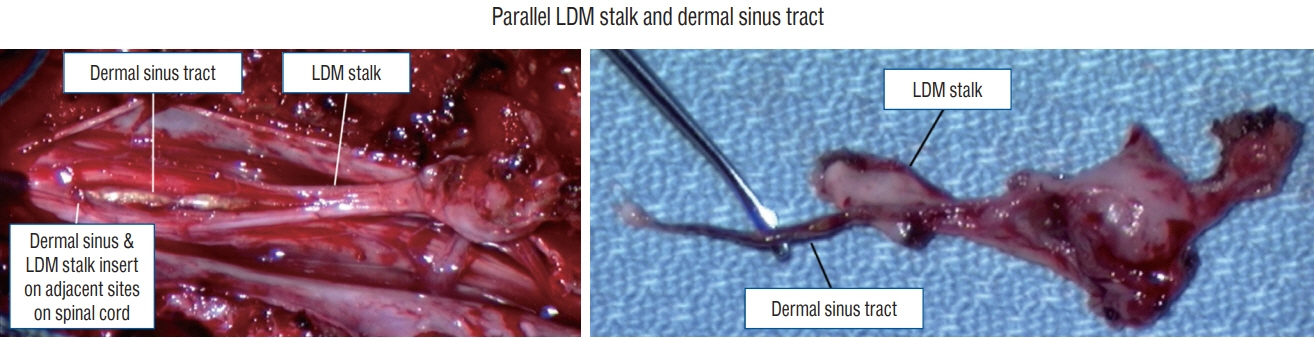
Fig. 44. Intraoperative photos showing the presence of a LDM and a CSDST in parallel. LDM : limited dorsal myeloschisis, CSDST : congenital spinal dermal sinus tract.
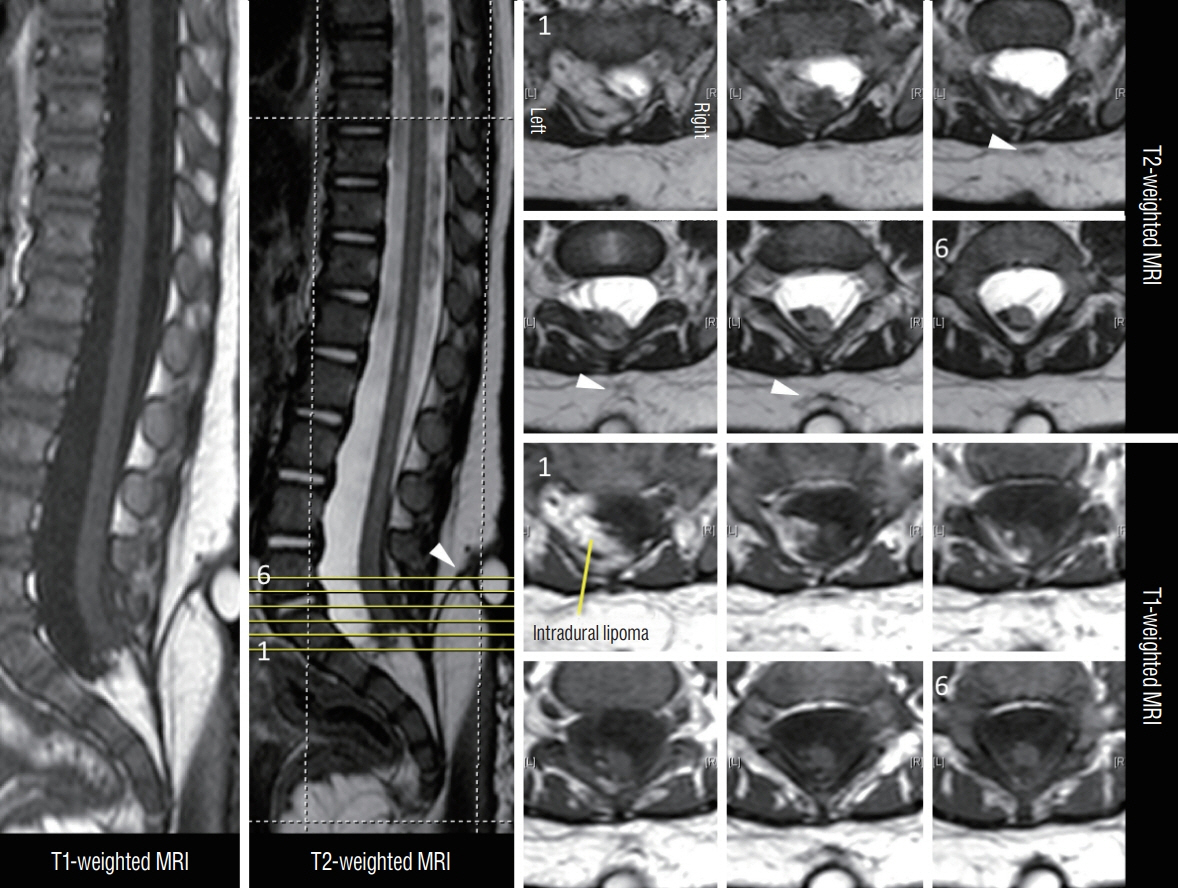
Fig. 45. MRI images showing a LDM associated with a transitional spinal cord lipoma in an 11-month-old. The axial cuts are numbered according to the cut-lines on the T2-weighted mid-sagittal MRI image. Arrowheads mean the subcutaneous portion of the LDM stalk. The stalk was confirmed intraoperatively to pass through bifid S1 and S2 laminae. Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging, LDM : limited dorsal myeloschisis.
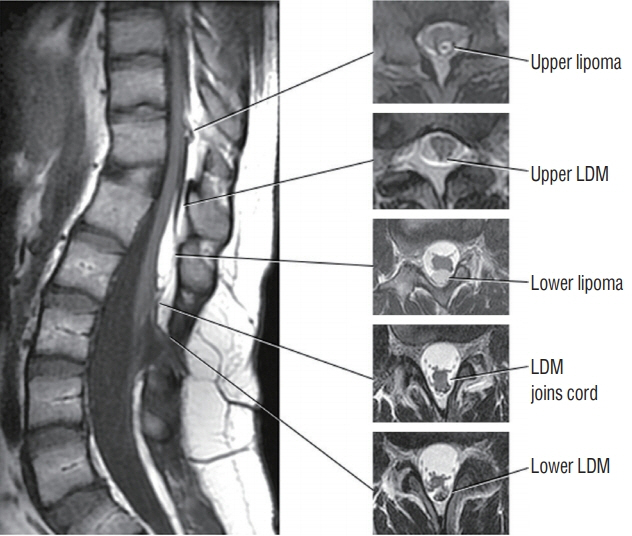
Fig. 46. Double LDMs, both crater type, with accompanying dorsal lipomas. The lower LDM is at L2/3, and upper LDM is at T12. Both accompanying lipomas are just rostral to the LDM stalk. Reused from Pang et al. [25] with permission from Springer Nature. LDM : limited dorsal myeloschisis.

Fig. 47. Intra-operative photographs showing excision of a dermal sinus tract (DST) with skip laminectomy technique (MRI images of this patient are shown in Figs. 4 and 5). B-G : Caudal. H-K : Rostral wound. A : Skin preparation. Yellow lines mean skin incisions. Numbers in black on skin mean levels of lumbar spinous processes. B : L4L5 laminectomy has been done. The subcutaneous portion of the DST (DSTsc) merging with the dura has been fully exposed. C : Photography taken after opening the dural sheath enveloping a portion of DST that is lying in the dura mater (DSTdu). D : Photography showing the arachnoid membrane entry site of the DST. E : After opening the arachnoid membrane, the intradural portion of the DST (DSTintradural) and keratin material are seen. F : The DSTdu has been removed. The DSTintradural is seen adhering to the filum. G : The caudal portion of the DST has been completely removed via the L4L5 laminectomy. H and I : Operative exposure via a T12L1 laminectomy. (H) is the T12 side of the exposure; (I) the L1 side. A dermoid cyst along a slender DST (DSTrostral) is shown in (I). The DSTrotral was then cut at the yellow cross, and the cyst with the portion of DST under the intact L2 and L3 laminae delivered from this exposure. The sub-millimeter thickness of the DST testifies to the difficulty in detecting them with MRI. J and K : Complete removal of the deep end of the DST from the dorsal midline of the spinal cord. Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging.
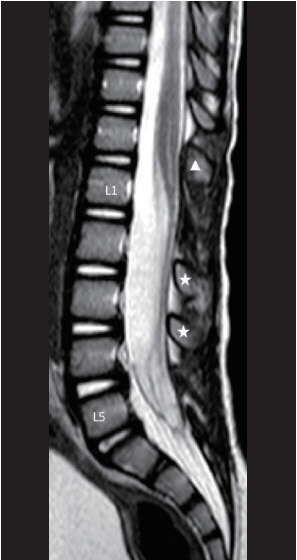
Fig. 48. Mid-sagittal MRI image showing the post-operative appearance of a skip laminectomy technique in which T12 laminoplasty, L1 laminectomy, and L4L5 laminectomy were done. White arrowhead means laminoplasty level. White stars mean intact laminae level. Reused from Wong et al. [43] with permission from Springer Nature.
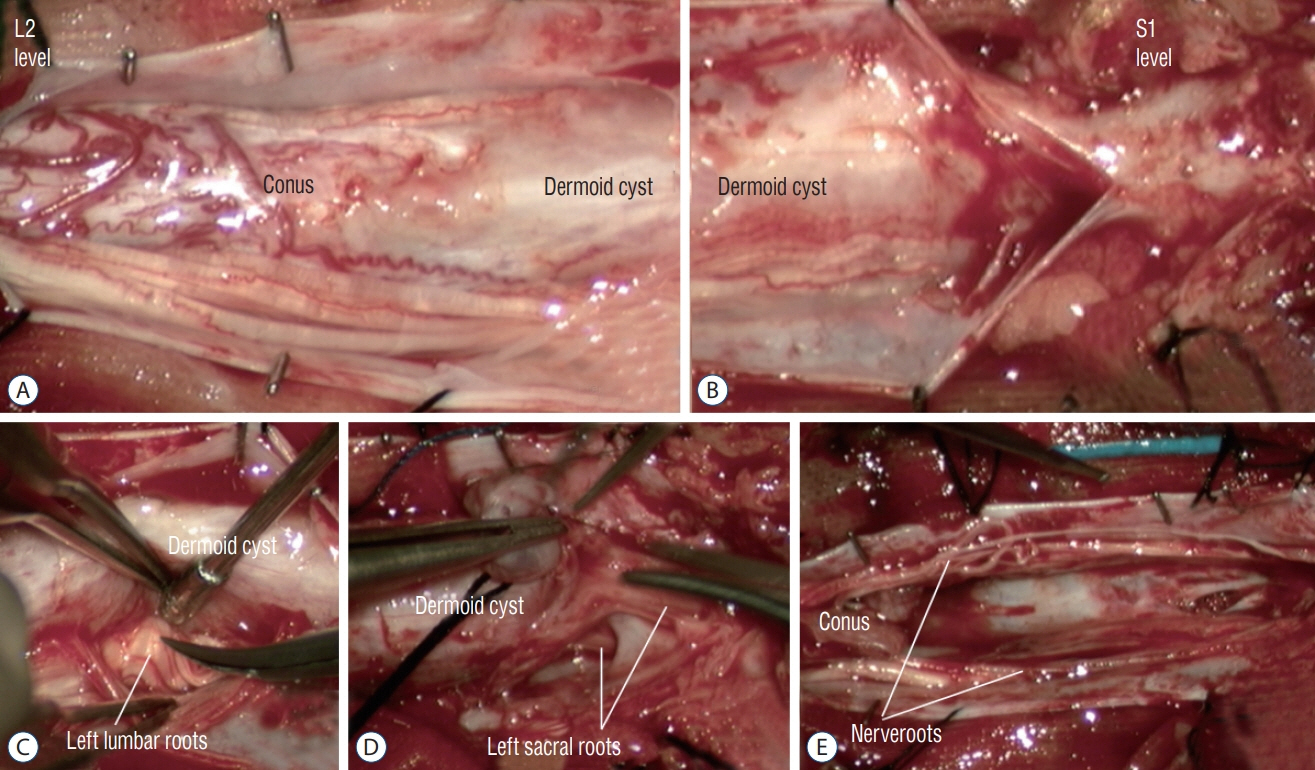
Fig. 49. Intra-operative photographs showing complete excision of an intradural dermoid cyst via L2–L5 laminoplasties and S1 laminectomy (MRI images are shown in Fig. 7). A and B : Surgical view after opening of dura. The dermoid cyst appears to merge with the conus and nerve roots. The dermoid cyst communicates with the skin ostium via a dermal sinus tract at the S1 laminar level. C and D : Dissection to free the nerve roots from the wall of the dermoid cyst. E : Excision cavity after complete excision of the dermoid cyst. Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging.
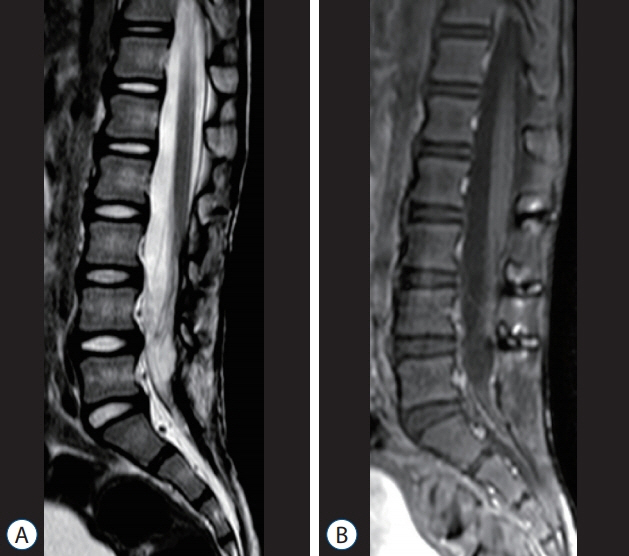
Fig. 50. Six-year post-operative MRI images (of the patient shown in Figs. 7 and 49) showing no recurrence of the dermoid cyst, and postlaminoplasty changes. A : T2-weighted MRI. B : T1-weighted MRI with gadolinium injection. Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging.
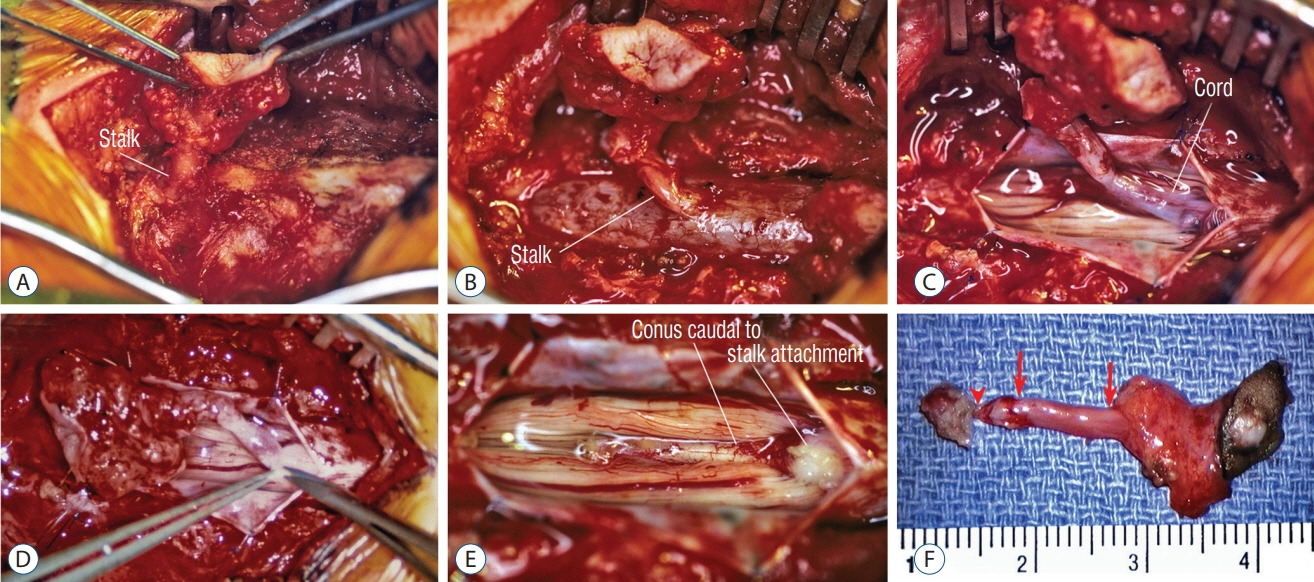
Fig. 51. Surgical resection of a lumbar crater-type flat (non-saccular) limited dorsal myeloschisis. A : Ellipse of resected skin crater and subcutaneous tract going through defect in lumbodorsal fascia. B : Extradural stalk and dural fistula. C : Intradural exposure showing stalk-cord union. D : Resection of stalk flush with cord surface. E : Normal conus caudal to stalk attachment site. F : En bloc specimen showing, from right to left, skin ellipse bearing pale epithelial crater, the subcutaneous portion bearing fat, the extradural portion of the stalk (between arrows), intradural stalk (between arrow and arrowhead), and an exuberant cuff of tissue on the cord. Reused from Pang et al. [25] with permission from Springer Nature.
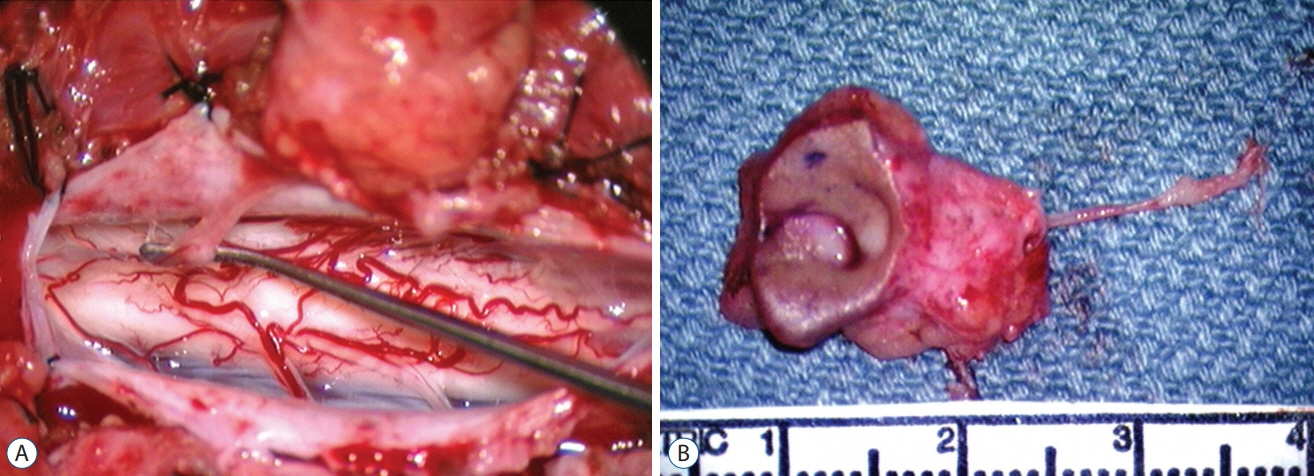
Fig. 52. Exceedingly slender limited dorsal myeloschisis (LDM) stalk. A : Stalk attaches to discrete spot on dorsal cord surface. B : En bloc specimen shows large complicated skin crater and the very slender LDM Stalk. Reused from Pang et al. [25] with permission from Springer Nature.
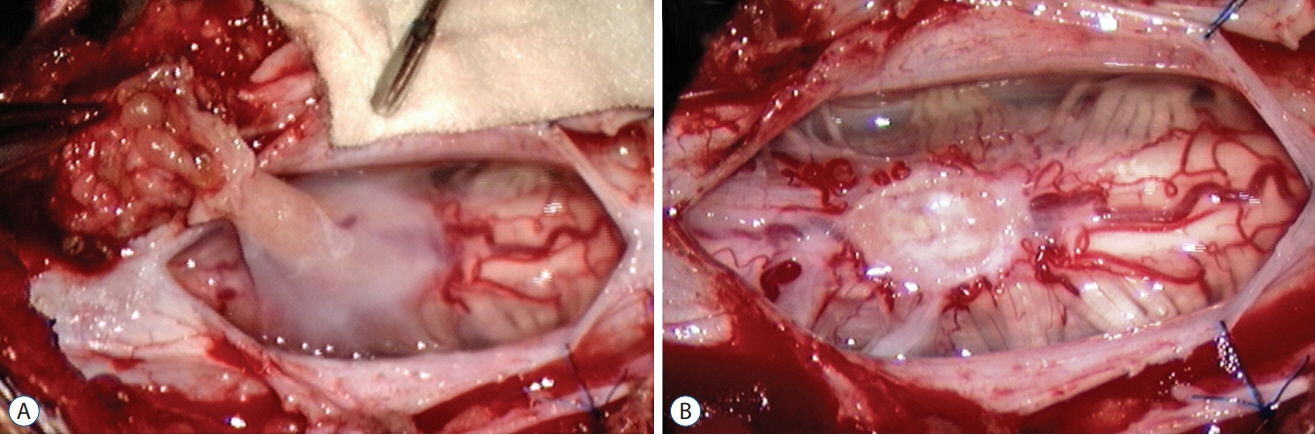
Fig. 53. Moderate-sized limited dorsal myeloschisis stalk. A : Stalk has a flared-out cord attachment. B : Stalk resection leaves a fish-mouth shaped scar. Reused from Pang et al. [25] with permission from Springer Nature.
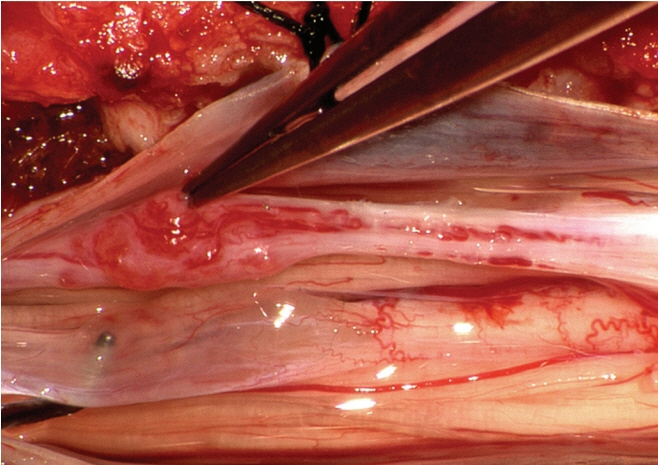
Fig. 54. Long limited dorsal myeloschisis stalk with vascular glomus (at tip of forceps). Reused from Pang et al. [25] with permission from Springer Nature.
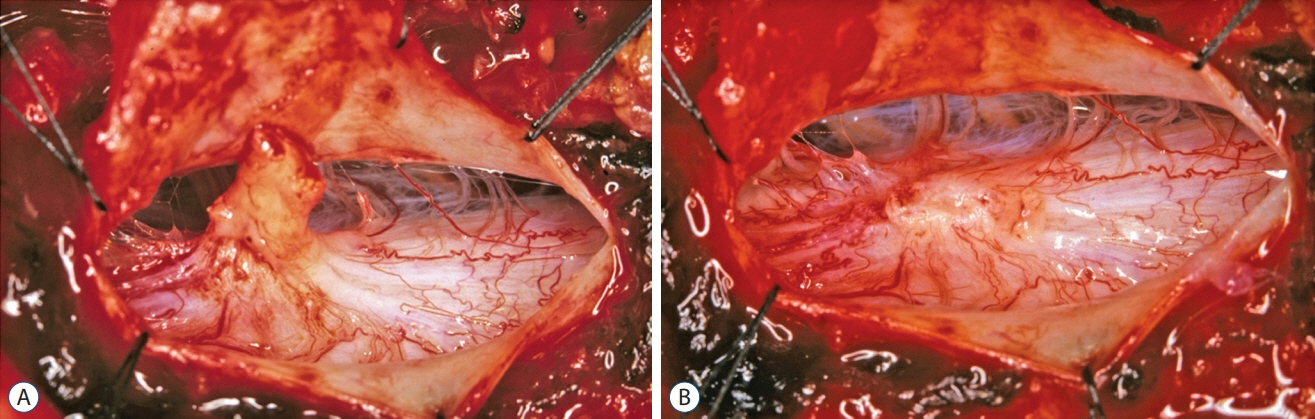
Fig. 55. Moderately thick limited dorsal myeloschisis (LDM) stalk with exuberant tentacles of blood vessels that seem to crawl on to the dorsal surface of the spinal cord. A : Before LDM resection. B : After LDM stalk resection showing centripetal distribution of the blood vessels. Reused from Pang et al. [25] with permission from Springer Nature.
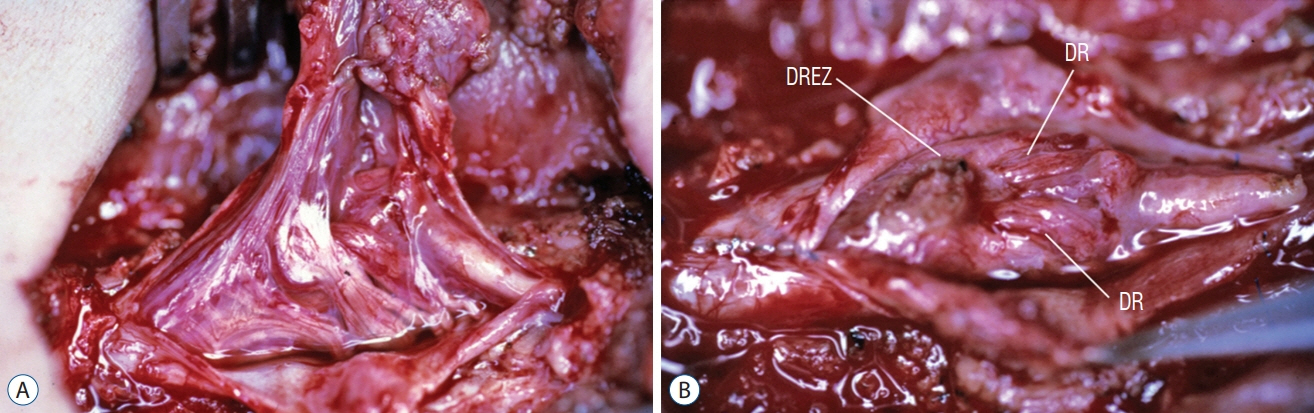
Fig. 56. Thick, complex-looking fibroneural stalk in a lumbar non-saccular LDM. A : Lesion contains dysplastic spinal cord tissue, large blood vessels, ample skein of non-functioning nerves, and thickened folded membranes. Spinal cord is lifted dorsally by the tethering effect. B : Bizarre arrangement of dorsal roots (DR), and dorsal root entry zone (DREZ) surround a large flat myeloschistic scar after stalk resection. Reused from Pang et al. [25] with permission from Springer Nature.
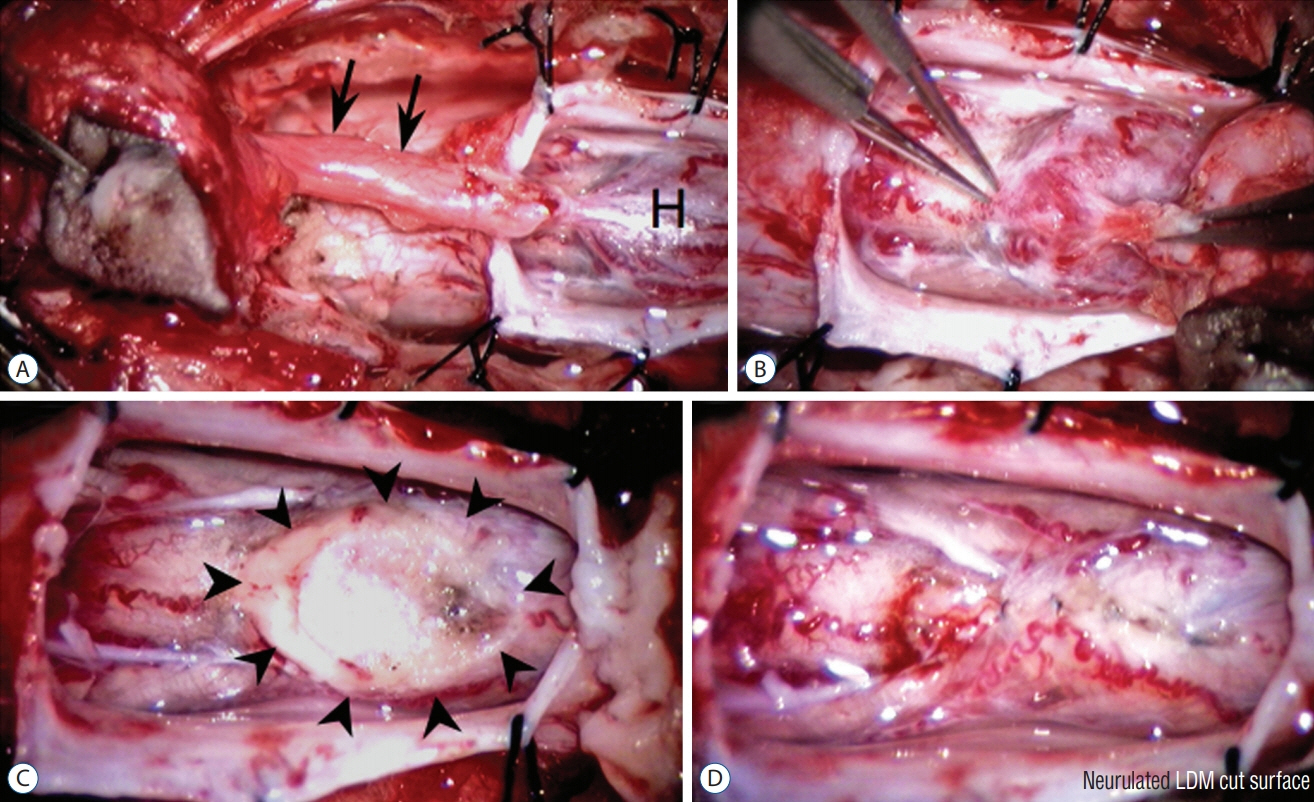
Fig. 57. Crater type lumbar limited dorsal myeloschisis (LDM). A : Lumbar LDM stalk (arrows) with a very prominent hump (H) of abnormal tissues on the cord. B : Resection at the base of this hump. C : Resection produced a very large scar (outlined by arrowheads). D : Dorsal pia-to-pia approximation of scar with 8-O Nylon sutures. Reused from Pang et al. [25] with permission from Springer Nature.
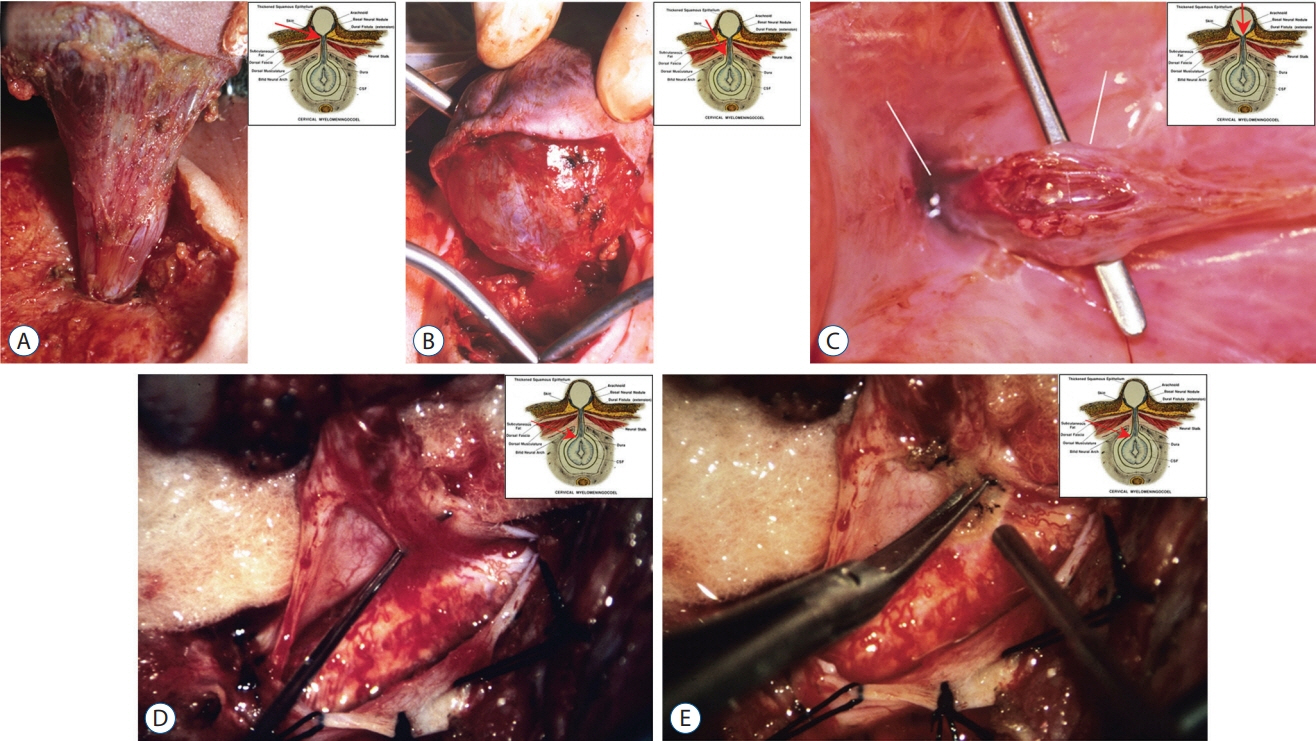
Fig. 58. Large cervical saccular limited dorsal myeloschisis. A : Exposure of the dorsal fistula at the level of the nuchal fascial defect. B : The neck of the sac passing through large laminar defect. C : The sac is opened from the top; basal neural nodule and the dural fistula opening (into the cyst) are seen through the cavity. D : Dural fistula opened into the main thecal sac, showing the thin fibroneural stalk inserting on to the dorsal spinal cord. E : Stalk being resected. All insets show exact level of the exposure. Reused from Pang et al. [25] with permission from Springer Nature.
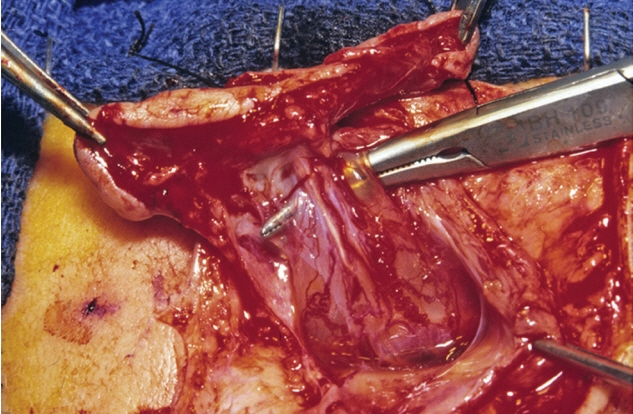
Fig. 59. Thoracic Saccular limited dorsal myeloschisis with the fibroneural stalk (displayed by instrument) traversing the sac cavity and reaching the dome of the sac. Reused from Pang et al. [25] with permission from Springer Nature.
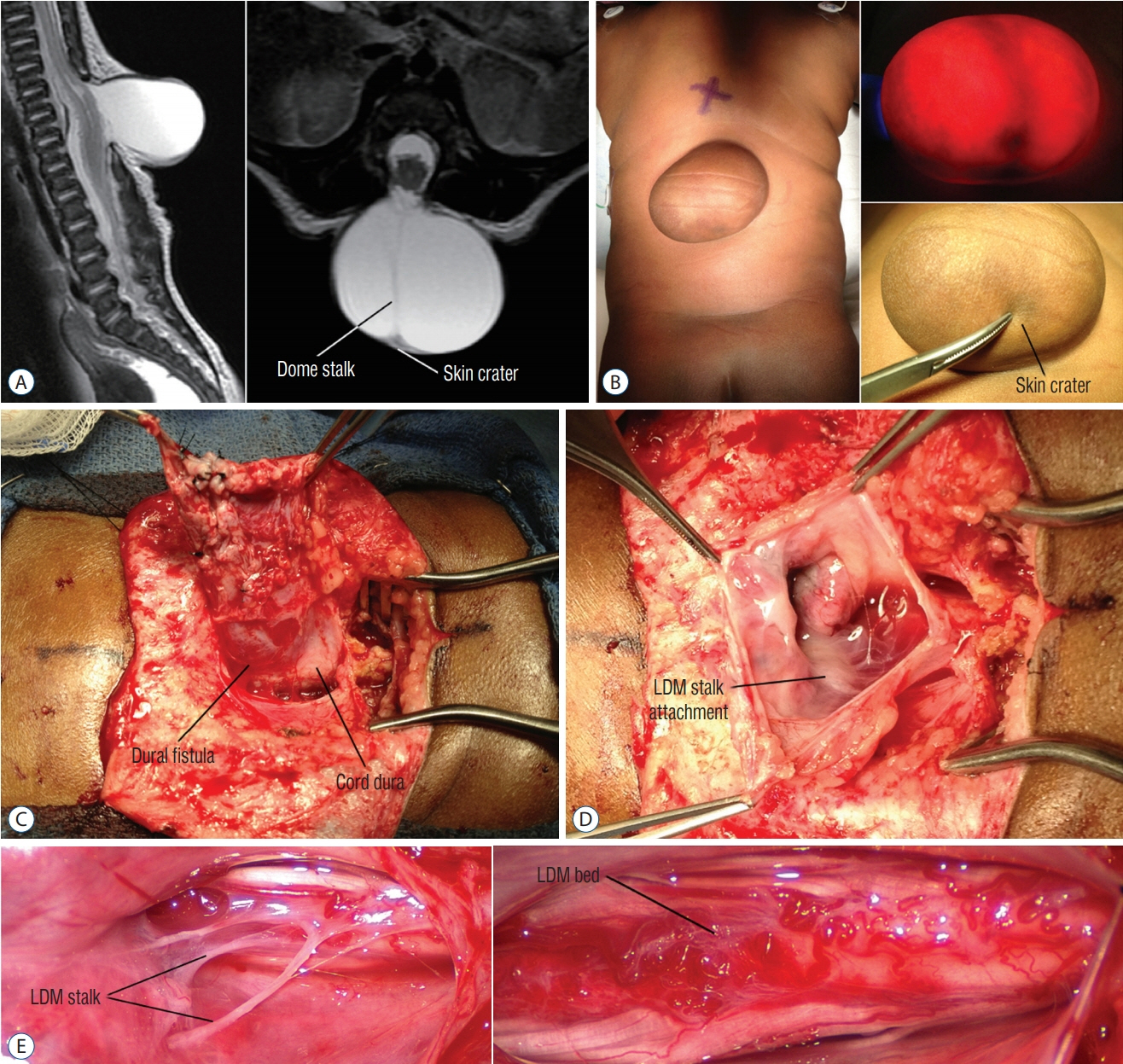
Fig. 60. Saccular limited dorsal myeloschisis (LDM) with the stalk-to-dome subtype of fibroneural stalk attachment. A : T2 magnetic resonance imaging shows slight tenting of the cord towards the sac on the sagittal image, and the fibroneural stalk (dome stalk) traversing the sac to the cord from the base of the skin crater in the axial image. B : The sac with the slightly darker irregular skin at the lower dome with a slightly thinner covering (skin crater, lower right) that may be thick squamous epithelium. The transilluminated picture (upper right) shows the small nubbin of (neural) tissue beneath the skin crater, and the stream of bands traversing the middle of the sac. C : Shows the wide neck of the dural fistula at the base of the sac, and its relationship with the cord dura. D : Sac opened from the top, showing the white area where the LDM stalk attaches to the cord surface. E : Close-up to show the strands of the LDM stalk inserting on the dorsal cord surface (upper). After resecting these strands (lower), the cord surface shows abnormal clusters of wiggly blood vessels and scar tissue. Reused from Pang et al. [25] with permission from Springer Nature.
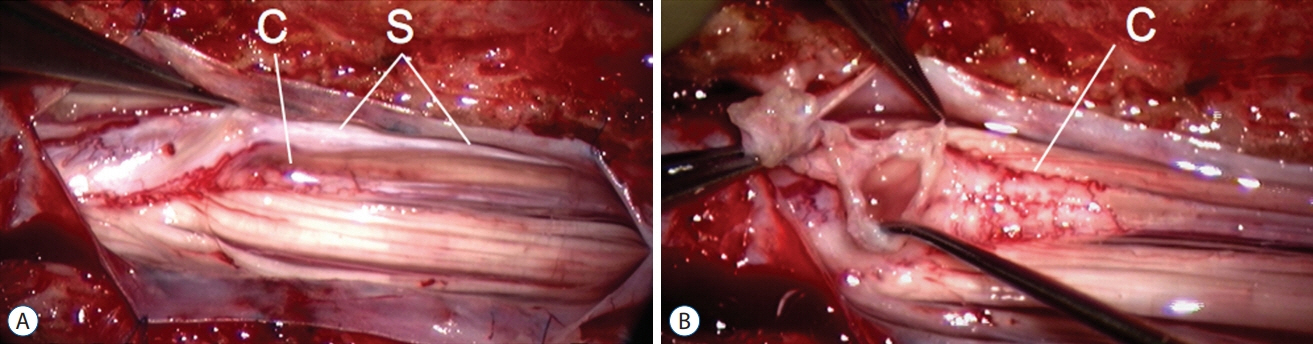
Fig. 61. Lumbar saccular limited dorsal myeloschisis with segmental myelocystocoele. A : A long intradural stalk (S) picked up by micro-forceps. B : Stalk traced to the hydromyelic portion of the cord after partial stalk resection. Reused from Pang et al. [25] with permission from Springer Nature. C : conus.
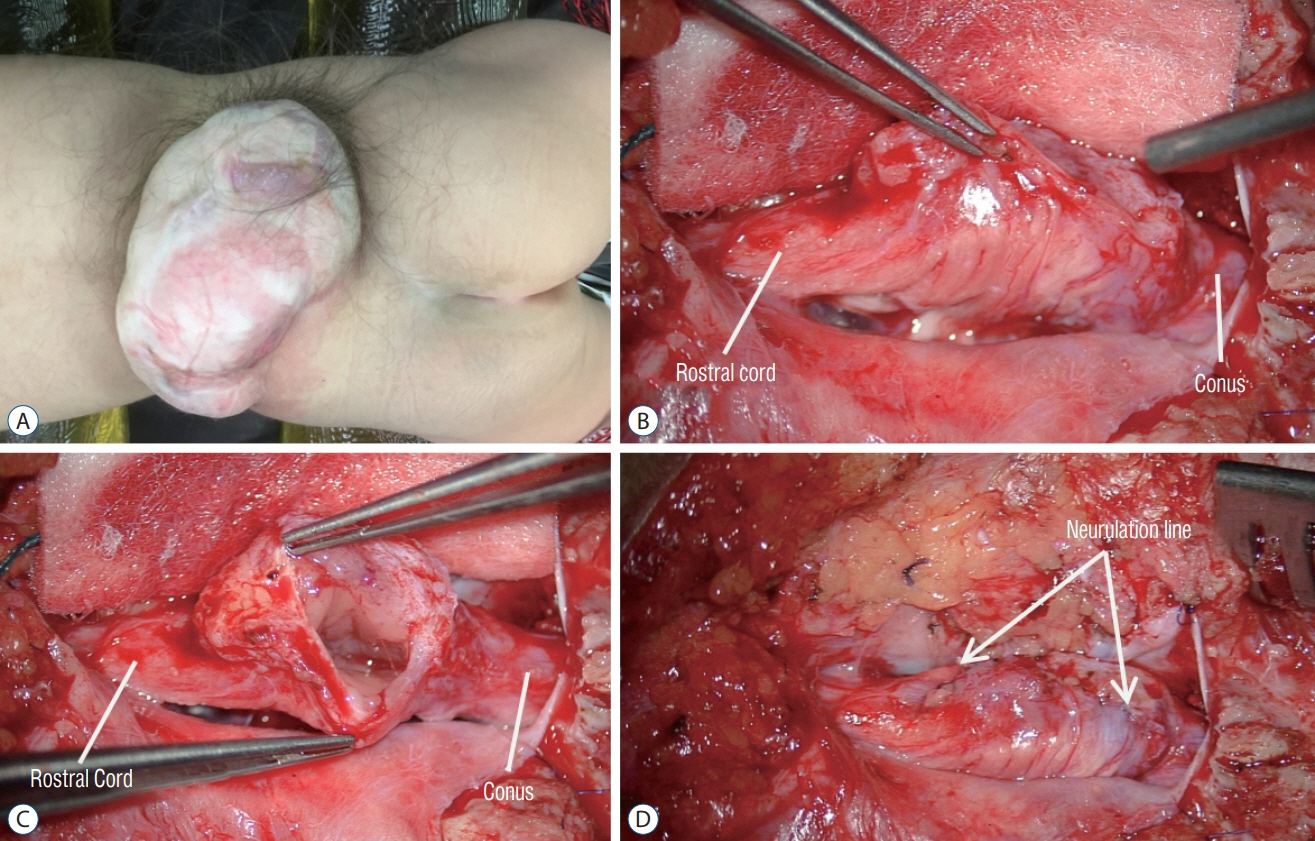
Fig. 62. Intraoperative photos of the case shown in Fig. 19. A : A large skin-based sac with pearly epithelium, and hypertrichosis around the base of the sac. B : The non-functional top of the cystocoele has been cut off leaving the functional base with nerve roots. C : Operative view looking through the neck of the cystocoele into the hydromyelic cavity. D : After neurulation closure of the myelocystocoele neck.
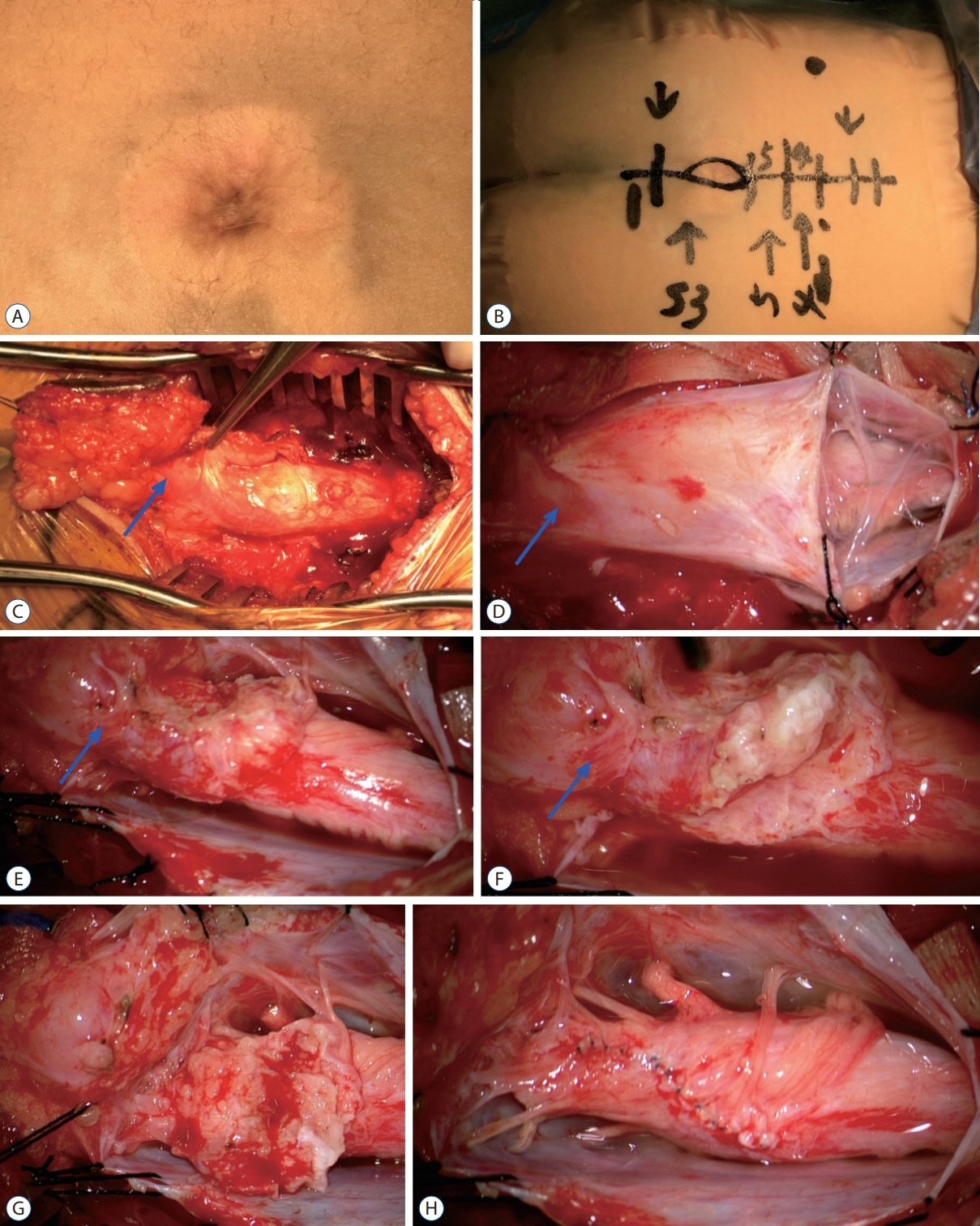
Fig. 63. Intra-operative photographs showing the excision of a LDM – lipoma complex (MRI images are shown in Fig. 45). A : The skin lesion, a cigar burn crater with surrounding skin discoloration. B : Markings for the skin incision. C : Surgical exposure after L4–S2 laminectomies. D : The dura has been opened. E : Surgical exposure after completion of “crotch dissection”. F : Detaching the stout LDM stalk and lipoma from the spinal cord. G : Residual fat on the placode before final trimming. H : Appearance of the spinal cord after neurulation. The nerve roots at the end of the spinal cord were stimulation positive. Blue arrow means Extradural portion of the LDM. The patient has no neurological deficits before and after the untethering surgery. Reused from Wong et al. [43] with permission from Springer Nature. LDM : limited dorsal myeloschisis, MRI : magnetic resonance imaging.
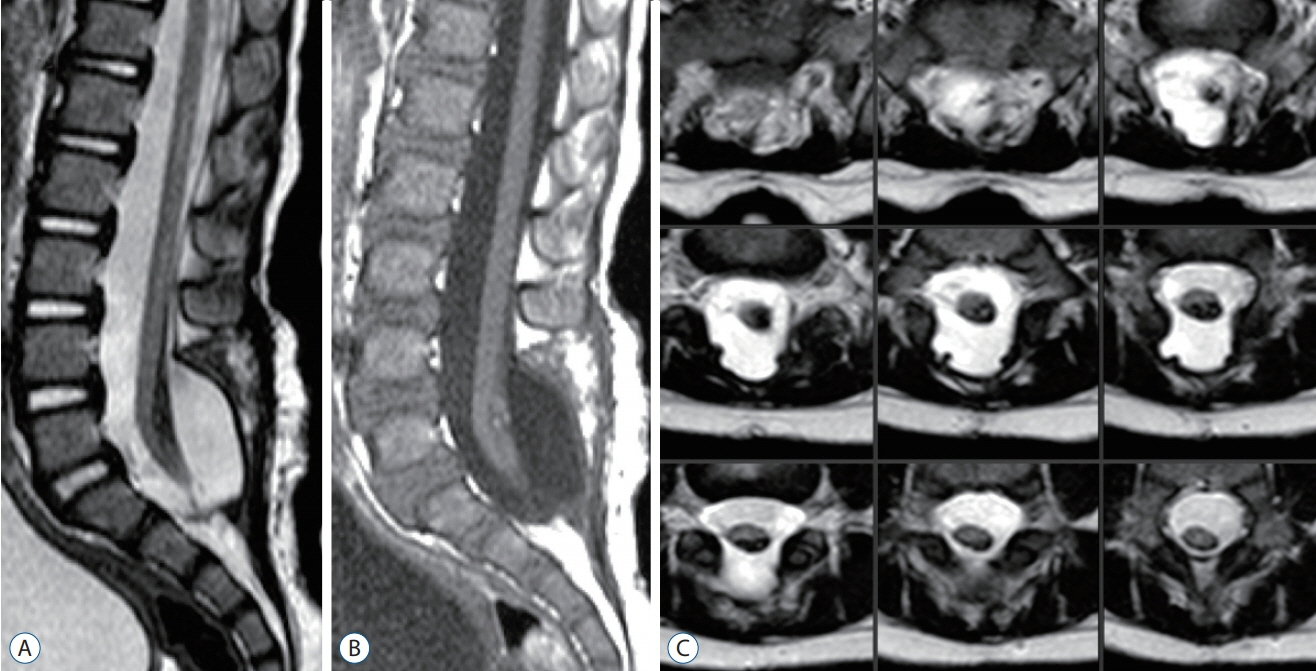
Fig. 64. Post-operative MRI images of the patient shown in Fig. 63. A : T2-weighed sagittal MRI image showing a cord-sac ratio of 33%. B : T1-weighted sagittal MRI image. C : Serial T2-weighted axial MRI images showing the spinal cord completely surrounded by cerebrospinal fluid. Reused from Wong et al. [43] with permission from Springer Nature. MRI : magnetic resonance imaging.

































































