Endocrinol Metab.
2021 Feb;36(1):146-156. 10.3803/EnM.2021.879.
Suppression of Fibrotic Reactions of Chitosan-Alginate Microcapsules Containing Porcine Islets by Dexamethasone Surface Coating
- Affiliations
-
- 1Department of Endocrinology and Metabolism, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Polymer Nano Science and Technology, Department of BIN Fusion Technology and BK-21 Polymer BIN Fusion Research Team, Chonbuk National University, Jeonju, Korea
- 3Department of Microbiology and Immunology, Translational Xenotransplantation Research Centre, Cancer Research Institute, Biomedical Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Endocrinology and Metabolism, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2513298
- DOI: http://doi.org/10.3803/EnM.2021.879
Abstract
- Background
The microencapsulation is an ideal solution to overcome immune rejection without immunosuppressive treatment. Poor biocompatibility and small molecular antigens secreted from encapsulated islets induce fibrosis infiltration. Therefore, the aims of this study were to improve the biocompatibility of microcapsules by dexamethasone coating and to verify its effect after xenogeneic transplantation in a streptozotocin-induced diabetes mice.
Methods
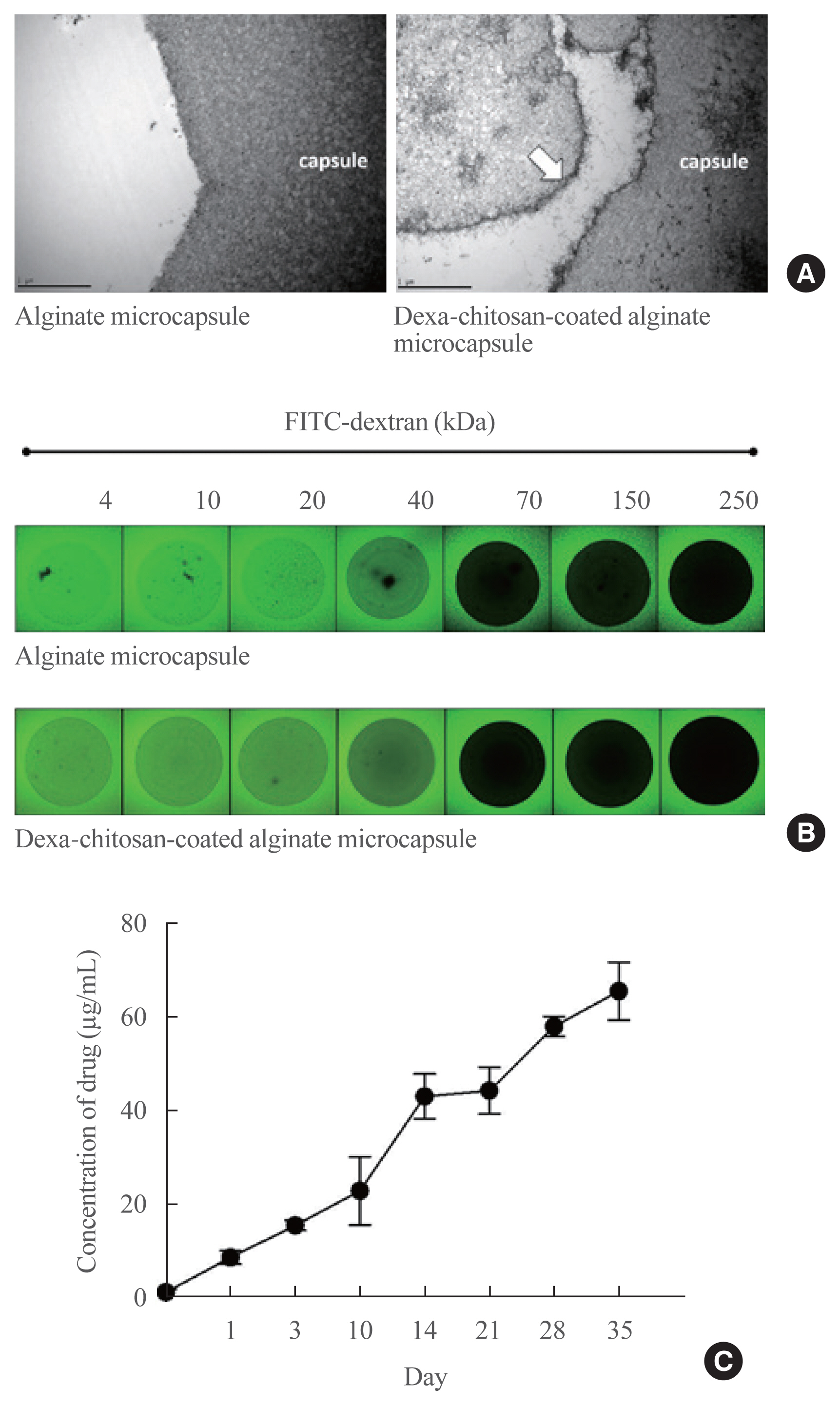
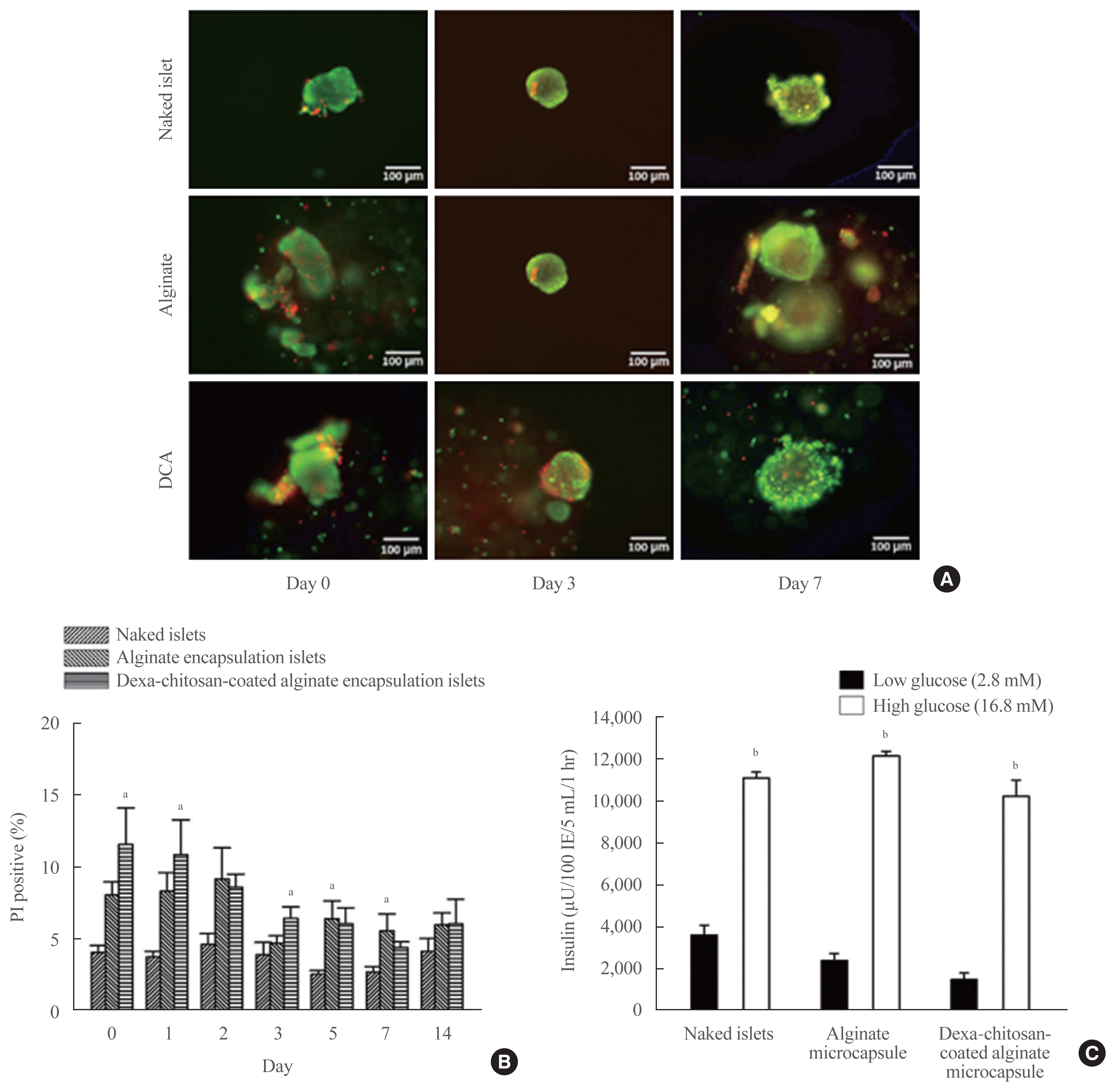
Dexamethasone 21-phosphate (Dexa) was dissolved in 1% chitosan and was cross-linked with the alginate microcapsule surface. Insulin secretion and viability assays were performed 14 days after microencapsulation. Dexa-containing chitosan-coated alginate (Dexa-chitosan) or alginate microencapsulated porcine islets were transplanted into diabetic mice. The fibrosis infiltration score was calculated from the harvested microcapsules. The harvested microcapsules were stained with trichrome and for insulin and macrophages.
Results
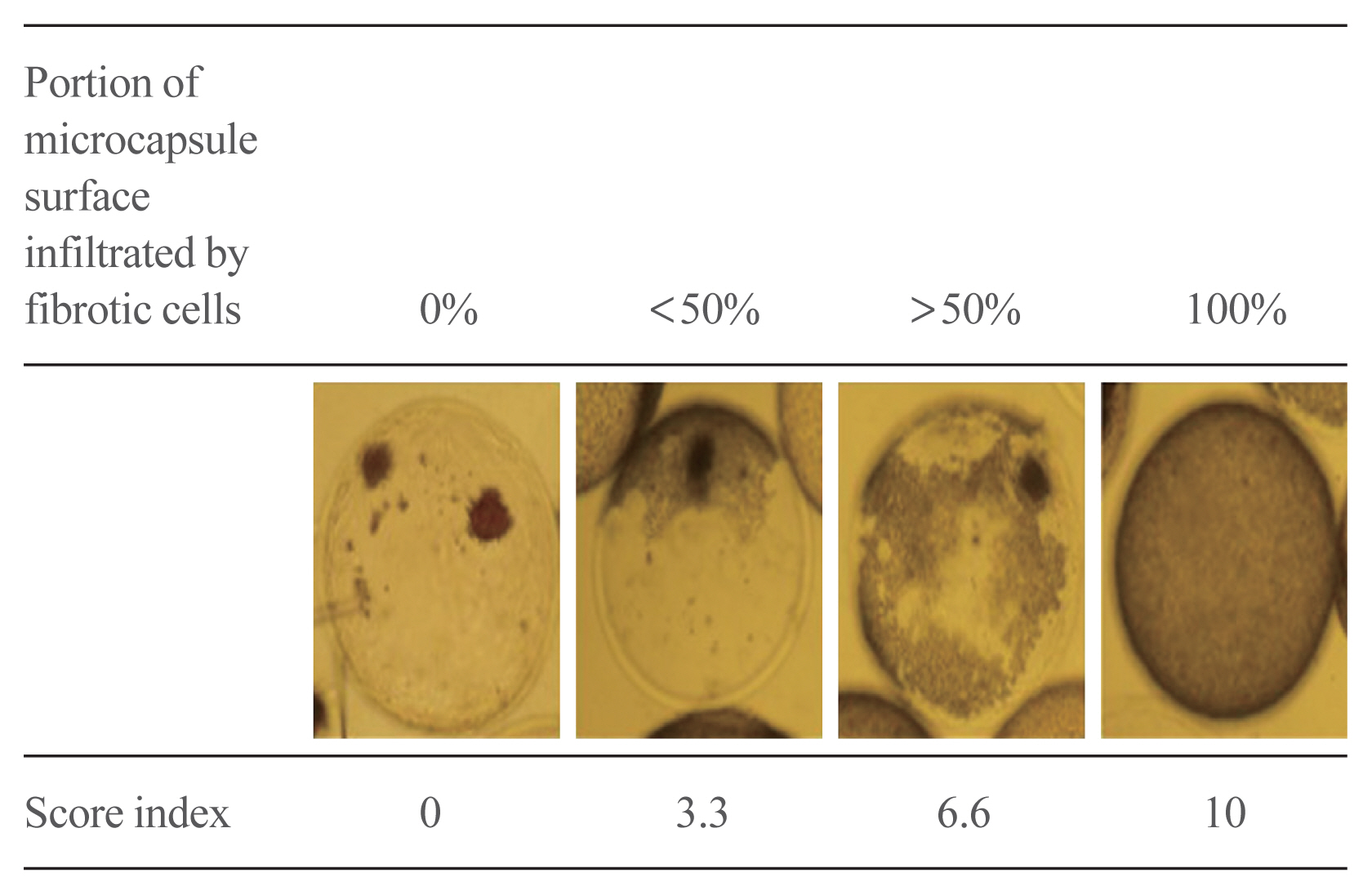
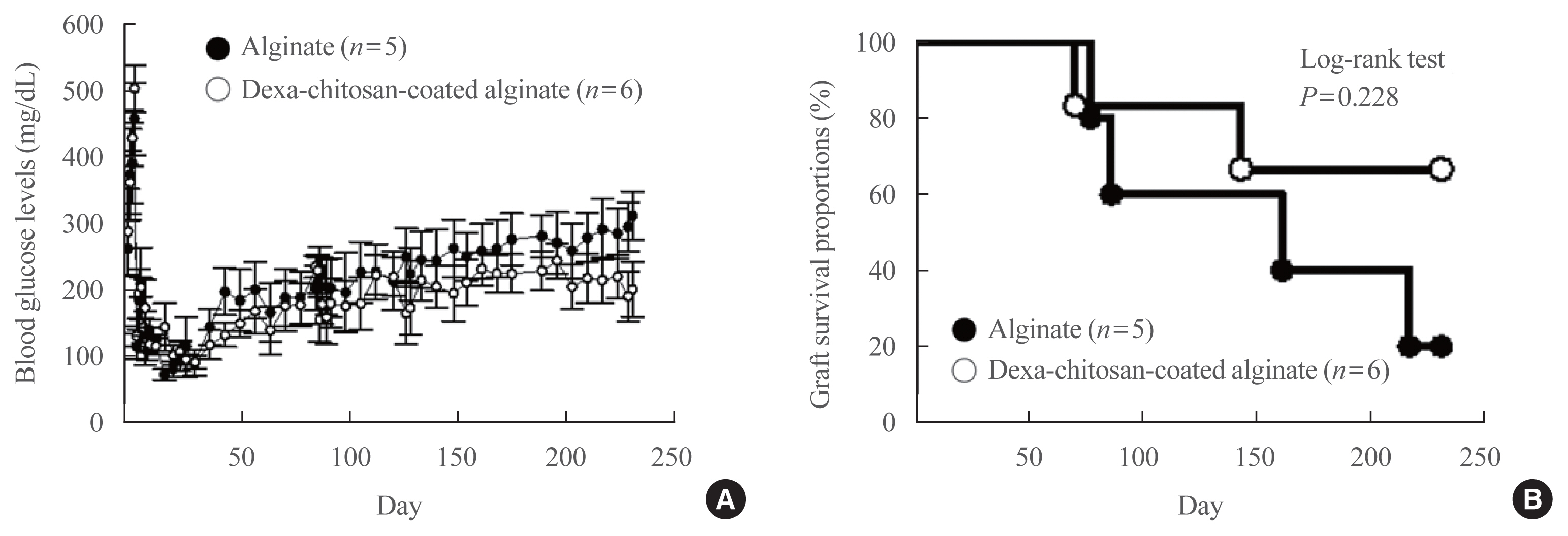
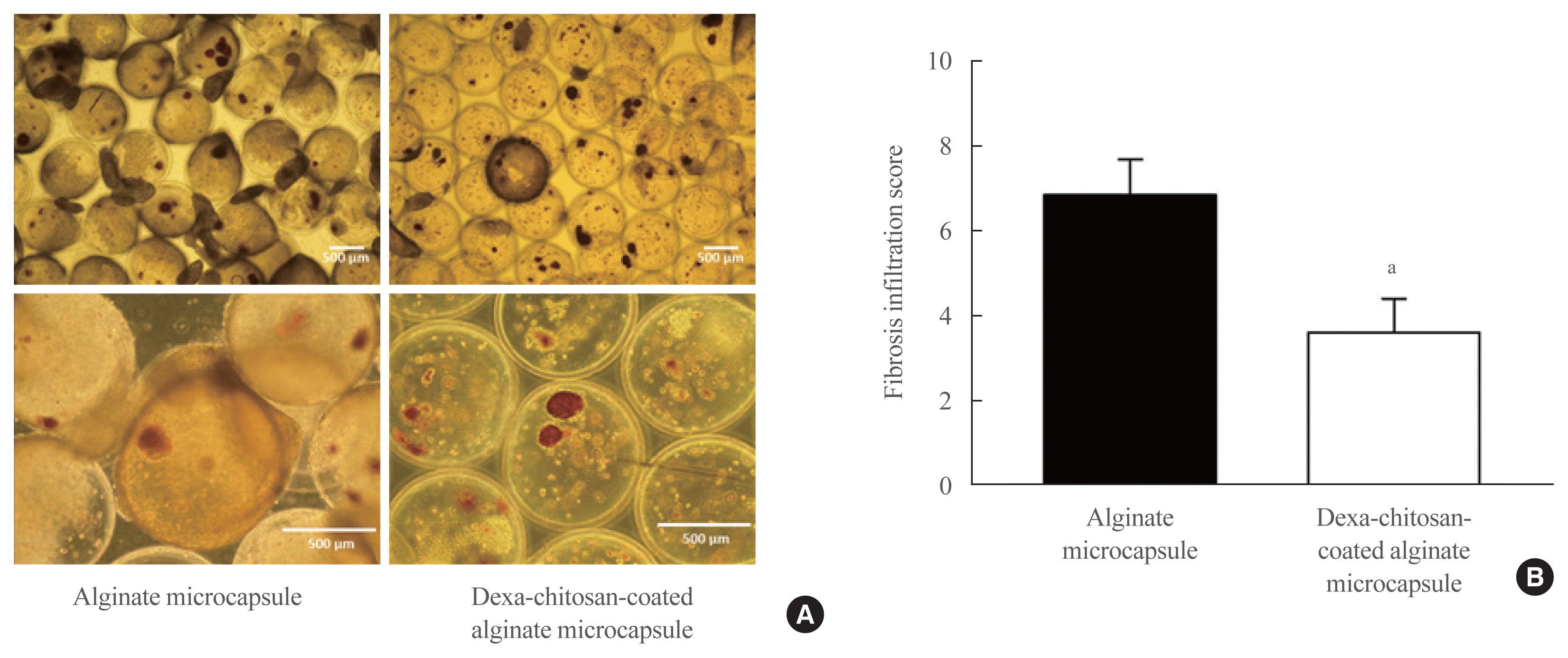
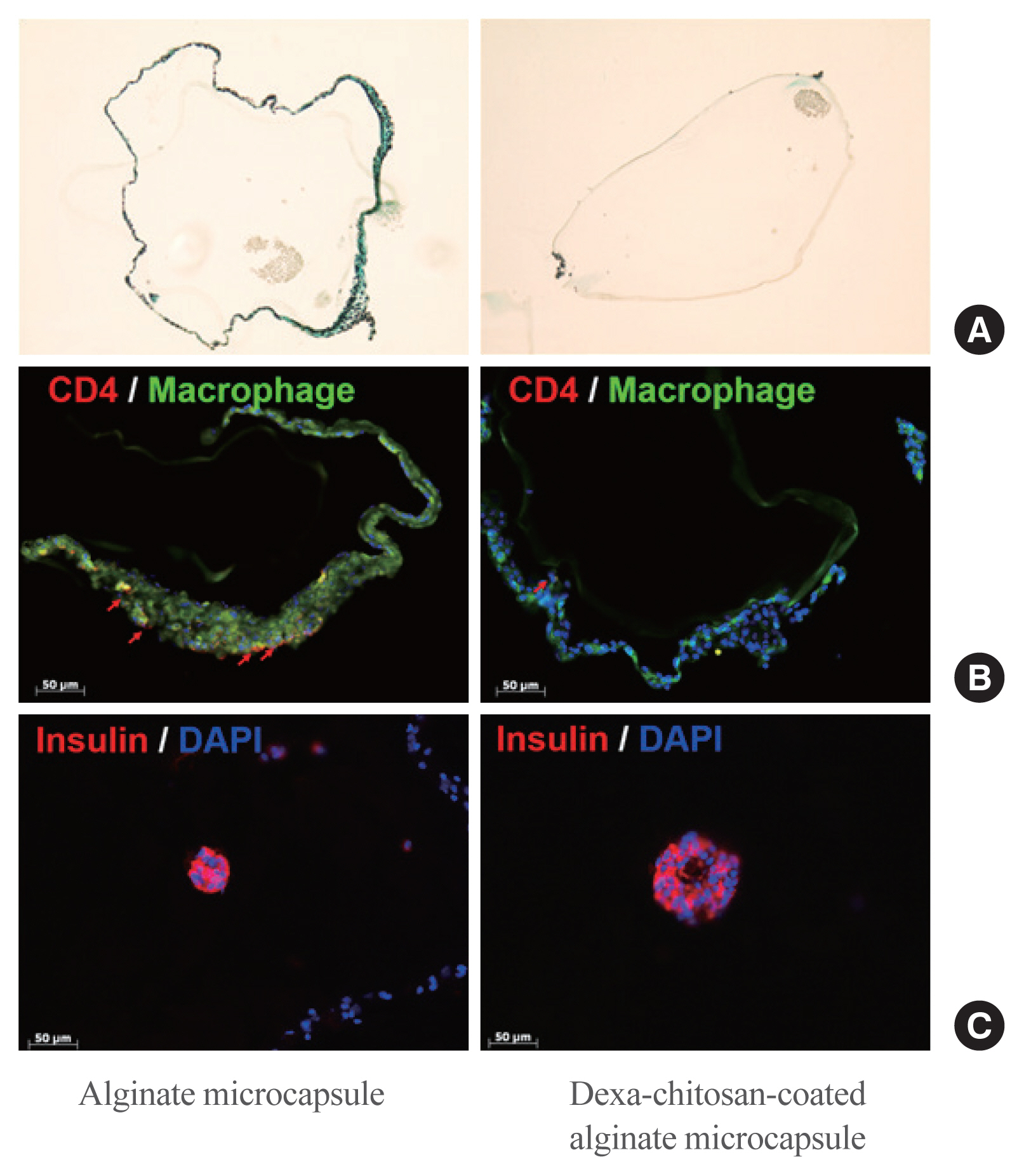
No significant differences in glucose-stimulated insulin secretion and islet viability were noted among naked, alginate, and Dexa-chitosan microencapsulated islets. After transplantation of microencapsulated porcine islets, nonfasting blood glucose were normalized in both the Dexa-chitosan and alginate groups until 231 days. The average glucose after transplantation were lower in the Dexa-chitosan group than the alginate group. Pericapsular fibrosis and inflammatory cell infiltration of microcapsules were significantly reduced in Dexa-chitosan compared with alginate microcapsules. Dithizone and insulin were positive in Dexa-chitosan capsules. Although fibrosis and macrophage infiltration was noted on the surface, some alginate microcapsules were stained with insulin.
Conclusion
Dexa coating on microcapsules significantly suppressed the fibrotic reaction on the capsule surface after transplantation of xenogenic islets containing microcapsules without any harmful effects on the function and survival of the islets.
Keyword
Figure
Reference
-
1. Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001; 358:221–9.
Article2. Lipsett M, Aikin R, Castellarin M, Hanley S, Jamal AM, Laganiere S, et al. Islet neogenesis: a potential therapeutic tool in type 1 diabetes. Int J Biochem Cell Biol. 2006; 38:498–503.
Article3. Murdoch TB, McGhee-Wilson D, Shapiro AM, Lakey JR. Methods of human islet culture for transplantation. Cell Transplant. 2004; 13:605–17.
Article4. Sakata N, Sumi S, Yoshimatsu G, Goto M, Egawa S, Unno M. Encapsulated islets transplantation: past, present and future. World J Gastrointest Pathophysiol. 2012; 3:19–26.
Article5. Boker A, Rothenberg L, Hernandez C, Kenyon NS, Ricordi C, Alejandro R. Human islet transplantation: update. World J Surg. 2001; 25:481–6.
Article6. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343:230–8.
Article7. Collaborative Islet Transplant Registry. CITR Tenth Annual Reports [Internet]. Rockville: citr;2017. [cited 2020 Nov 25]. Available from: http://citregistry.org/content/reports-publications-presentations .8. Larsen JL, Bennett RG, Burkman T, Ramirez AL, Yamamoto S, Gulizia J, et al. Tacrolimus and sirolimus cause insulin resistance in normal Sprague Dawley rats. Transplantation. 2006; 82:466–70.
Article9. Dai C, Walker JT, Shostak A, Padgett A, Spears E, Wisniewski S, et al. Tacrolimus- and sirolimus-induced human β cell dysfunction is reversible and preventable. JCI Insight. 2020; 5:e130770.
Article10. Dufrane D, Gianello P. Macro- or microencapsulation of pig islets to cure type 1 diabetes. World J Gastroenterol. 2012; 18:6885–93.
Article11. Juste S, Lessard M, Henley N, Menard M, Halle JP. Effect of poly-L-lysine coating on macrophage activation by alginate-based microcapsules: assessment using a new in vitro method. J Biomed Mater Res A. 2005; 72:389–98.12. Lim DJ, Cho JH, Choi YH, Park HS, Hong OK, Kwon HS, et al. Optimization of microencapsulation of the Islets. Tissue Eng Regen Med. 2005; 2:280–6.13. Jiang K, Weaver JD, Li Y, Chen X, Liang J, Stabler CL. Local release of dexamethasone from macroporous scaffolds accelerates islet transplant engraftment by promotion of anti-inflammatory M2 macrophages. Biomaterials. 2017; 114:71–81.
Article14. Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006; 27:2450–67.
Article15. Labhasetwar V, Levy RJ. Implants for site-specific drug delivery. J Appl Biomater. 1991; 2:211–2.
Article16. Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, et al. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007; 83:585–96.
Article17. Ramalingam A, Hirai A, Thompson EA. Glucocorticoid inhibition of fibroblast proliferation and regulation of the cyclin kinase inhibitor p21Cip1. Mol Endocrinol. 1997; 11:577–86.
Article18. Shi K, Jiang J, Ma T, Xie J, Duan L, Chen R, et al. Dexamethasone attenuates bleomycin-induced lung fibrosis in mice through TGF-β, Smad3 and JAK-STAT pathway. Int J Clin Exp Med. 2014; 7:2645–50.19. Zawalich WS, Tesz GJ, Yamazaki H, Zawalich KC, Philbrick W. Dexamethasone suppresses phospholipase C activation and insulin secretion from isolated rat islets. Metabolism. 2006; 55:35–42.
Article20. Kaiser G, Gerst F, Michael D, Berchtold S, Friedrich B, Strutz-Seebohm N, et al. Regulation of forkhead box O1 (FOXO1) by protein kinase B and glucocorticoids: different mechanisms of induction of beta cell death in vitro. Diabetologia. 2013; 56:1587–95.
Article21. Kim JW, Sun C, Jeon SY, You YH, Shin JY, Lee SH, et al. Glucocorticoid treatment independently affects expansion and transdifferentiation of porcine neonatal pancreas cell clusters. BMB Rep. 2012; 45:51–6.
Article22. Jin SM, Shin JS, Kim KS, Gong CH, Park SK, Kim JS, et al. Islet isolation from adult designated pathogen-free pigs: use of the newer bovine nervous tissue-free enzymes and a revised donor selection strategy would improve the islet graft function. Xenotransplantation. 2011; 18:369–79.
Article23. O’Neil JJ, Stegemann JP, Nicholson DT, Gagnon KA, Solomon BA, Mullon CJ. The isolation and function of porcine islets from market weight pigs. Cell Transplant. 2001; 10:235–46.
Article24. Pignatello R, Stancampiano AH, Ventura CA, Puglisi G. Dexamethasone sodium phosphate-loaded Chitosan based delivery systems for buccal application. J Drug Target. 2007; 15:603–10.
Article25. Hall KK, Gattas-Asfura KM, Stabler CL. Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via Staudinger ligation. Acta Biomater. 2011; 7:614–24.
Article26. Sylvestre JP, Guy RH, Delgado-Charro MB. In vitro optimization of dexamethasone phosphate delivery by iontophoresis. Phys Ther. 2008; 88:1177–85.
Article27. Tam SK, Bilodeau S, Dusseault J, Langlois G, Halle JP, Yahia LH. Biocompatibility and physicochemical characteristics of alginate-polycation microcapsules. Acta Biomater. 2011; 7:1683–92.
Article28. Vergani A, D’Addio F, Jurewicz M, Petrelli A, Watanabe T, Liu K, et al. A novel clinically relevant strategy to abrogate autoimmunity and regulate alloimmunity in NOD mice. Diabetes. 2010; 59:2253–64.
Article29. Merani S, Shapiro AM. Current status of pancreatic islet transplantation. Clin Sci (Lond). 2006; 110:611–25.
Article30. Park HS, Ham DS, You YH, Shin JY, Kim JW, Jo JH, et al. Successful xenogenic islet transplantation with Ba2+-alginate encapsulation. Tissue Eng Regen Med. 2010; 7:523–30.31. Singarayar S, Kistler PM, De Winter C, Mond H. A comparative study of the action of dexamethasone sodium phosphate and dexamethasone acetate in steroid-eluting pacemaker leads. Pacing Clin Electrophysiol. 2005; 28:311–5.
Article32. Schneider BL, Schwenter F, Pralong WF, Aebischer P. Prevention of the initial host immuno-inflammatory response determines the long-term survival of encapsulated myoblasts genetically engineered for erythropoietin delivery. Mol Ther. 2003; 7:506–14.
Article33. Zhang WJ, Marx SK, Laue C, Hyder A, Juergensen A, Bickel M, et al. HOE 077 reduces fibrotic overgrowth around the barium alginate microcapsules. Transplant Proc. 2000; 32:206–9.
Article34. Dang TT, Thai AV, Cohen J, Slosberg JE, Siniakowicz K, Doloff JC, et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials. 2013; 34:5792–801.
Article35. Park HS, Kim JW, Lee SH, Yang HK, Ham DS, Sun CL, et al. Antifibrotic effect of rapamycin containing polyethylene glycol-coated alginate microcapsule in islet xenotransplantation. J Tissue Eng Regen Med. 2017; 11:1274–84.
Article36. Azadi SA, Vasheghani-Farahani E, Hashemi-Najafbabadi S, Godini A. Co-encapsulation of pancreatic islets and pentoxifylline in alginate-based microcapsules with enhanced immunosuppressive effects. Prog Biomater. 2016; 5:101–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preadipocyte Culture in Chitosan-Alginate Gel
- Alginate Microencapsulation of Islet Cells Using Electrostatic Droplet Generator
- Experimental Micro Encapsulation of Pancreatic Islets with Air-driven Droplet Generator and Alginate
- Effect of oral ingestion of chitosan and alginate on the removal of orally ingested radiostrontium(Sr) in mice
- Effects of Chitosan on Human Periodontal Ligament Cells in Vitro