Endocrinol Metab.
2021 Feb;36(1):22-29. 10.3803/EnM.2021.102.
Peptidyl and Non-Peptidyl Oral Glucagon-Like Peptide-1 Receptor Agonists
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2513284
- DOI: http://doi.org/10.3803/EnM.2021.102
Abstract
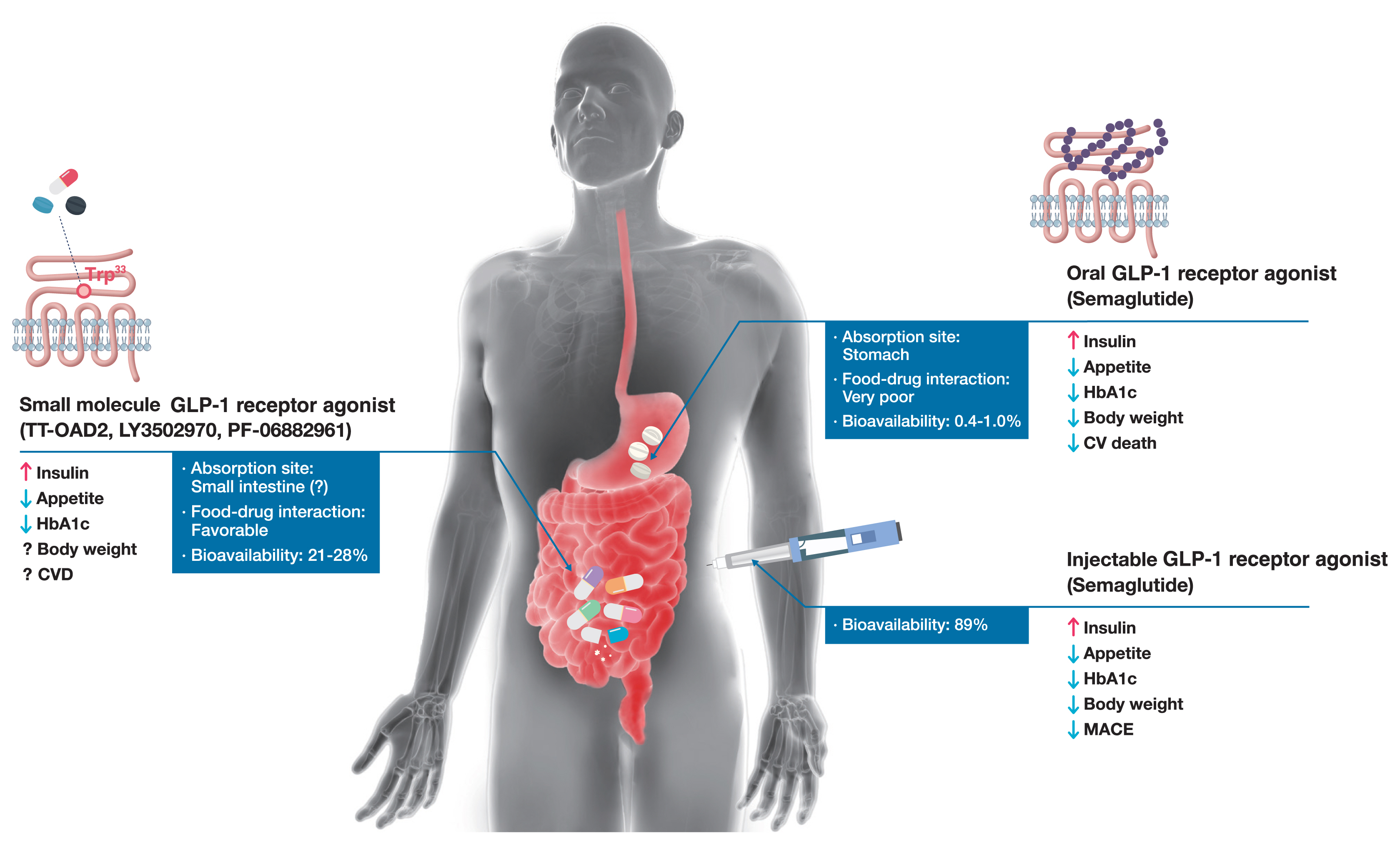
- Glucagon-like peptide-1 (GLP-1) receptor agonists are efficacious glucose-lowering medications with salient benefits for body weight and cardiovascular events. This class of medications is now recommended as the top priority for patients with established cardiovascular disease or indicators of high risk. Until the advent of oral semaglutide, however, GLP-1 receptor agonists were available only in the form of subcutaneous injections. Aversion to needles, discomfort with self-injection, or skin problems at the injection site are commonly voiced problems in people with diabetes, and thus, attempts for non-invasive delivery strategies have continued. Herein, we review the evolution of GLP-1 therapy from its discovery and the development of currently approved drugs to the unprecedented endeavor to administer GLP-1 receptor agonists via the oral route. We focus on the pharmacokinetic and pharmacodynamic properties of the recently approved oral GLP-1 receptor agonist, oral semaglutide. Small molecule oral GLP-1 receptor agonists are currently in development, and we introduce how these chemicals have addressed the challenge posed by interactions with the large extracellular ligand binding domain of the GLP-1 receptor. We specifically discuss the structure and pharmacological properties of TT-OAD2, LY3502970, and PF-06882961, and envision an era where more patients could benefit from oral GLP-1 receptor agonist therapy.
Keyword
Figure
Reference
-
1. Jorsal T, Rhee NA, Pedersen J, Wahlgren CD, Mortensen B, Jepsen SL, et al. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia. 2018; 61:284–94.
Article2. Cho YM, Fujita Y, Kieffer TJ. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu Rev Physiol. 2014; 76:535–59.
Article3. Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983; 302:716–8.
Article4. Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995; 136:3585–96.
Article5. Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992; 326:1316–22.
Article6. Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002; 359:824–30.7. Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom: further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992; 267:7402–5.
Article8. Ha JH, Kim JE, Kim YS. Immunoglobulin Fc heterodimer platform technology: from design to applications in therapeutic antibodies and proteins. Front Immunol. 2016; 7:394.
Article9. Del Prato S, Kang J, Trautmann ME, Stewart J, Sorli CH, Derwahl M, et al. Efficacy and safety of once-monthly efpeglenatide in patients with type 2 diabetes: results of a phase 2 placebo-controlled, 16-week randomized dose-finding study. Diabetes Obes Metab. 2020; 22:1176–86.
Article10. Cho YM, Merchant CE, Kieffer TJ. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacol Ther. 2012; 135:247–78.
Article11. Cho YM, Wideman RD, Kieffer TJ. Clinical application of glucagon-like peptide 1 receptor agonists for the treatment of type 2 diabetes mellitus. Endocrinol Metab (Seoul). 2013; 28:262–74.
Article12. Gutniak MK, Larsson H, Sanders SW, Juneskans O, Holst JJ, Ahren B. GLP-1 tablet in type 2 diabetes in fasting and postprandial conditions. Diabetes Care. 1997; 20:1874–9.
Article13. Leone-Bay A, Grant M, Greene S, Stowell G, Daniels S, Smithson A, et al. Evaluation of novel particles as an inhalation system for GLP-1. Diabetes Obes Metab. 2009; 11:1050–9.
Article14. Rosenstock J, Buse JB, Azeem R, Prabhakar P, Kjems L, Huang H, et al. Efficacy and safety of ITCA 650, a novel drug-device GLP-1 receptor agonist, in type 2 diabetes uncontrolled with oral antidiabetes drugs: the FREEDOM-1 trial. Diabetes Care. 2018; 41:333–40.
Article15. Lee SH, Min SH, Cho YC, Han JH, Kim MN, Kim CR, et al. Magnetically-driven implantable pump for on-demand bolus infusion of short-acting glucagon-like peptide-1 receptor agonist. J Control Release. 2020; 325:111–20.
Article16. Baughman RA, Kapoor SC, Agarwal RK, Kisicki J, Catella-Lawson F, FitzGerald GA. Oral delivery of anticoagulant doses of heparin: a randomized, double-blind, controlled study in humans. Circulation. 1998; 98:1610–5.17. Castelli MC, Wong DF, Friedman K, Riley MG. Pharmacokinetics of oral cyanocobalamin formulated with sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC): an open-label, randomized, single-dose, parallel-group study in healthy male subjects. Clin Ther. 2011; 33:934–45.
Article18. Twarog C, Fattah S, Heade J, Maher S, Fattal E, Brayden DJ. Intestinal permeation enhancers for oral delivery of macromolecules: a comparison between salcaprozate sodium (SNAC) and sodium caprate (C10). Pharmaceutics. 2019; 11:78.
Article19. Novo Nordisk Inc. RYBELSUS (semaglutide) [Internet]. Silver Spring;U.S. Food and Drug Administration: 2019. [cited 2021 Jan 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf .20. Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OK, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017; 318:1460–70.
Article21. Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SO, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019; 42:2272–81.22. Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019; 42:1724–1732.
Article23. Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019; 321:1466–80.
Article24. Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020; 22:1263–77.
Article25. Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019; 7:515–27.26. Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019; 394:39–50.
Article27. Buse JB, Bode BW, Mertens A, Cho YM, Christiansen E, Hertz CL, et al. Long-term efficacy and safety of oral semaglutide and the effect of switching from sitagliptin to oral semaglutide in patients with type 2 diabetes: a 52-week, randomized, open-label extension of the PIONEER 7 trial. BMJ Open Diabetes Res Care. 2020; 8:e001649.
Article28. Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CL, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019; 7:528–39.29. Zinman B, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff ST, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019; 42:2262–71.30. Yamada Y, Katagiri H, Hamamoto Y, Deenadayalan S, Navarria A, Nishijima K, et al. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020; 8:377–91.
Article31. Yabe D, Nakamura J, Kaneto H, Deenadayalan S, Navarria A, Gislum M, et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020; 8:392–406.
Article32. Avgerinos I, Michailidis T, Liakos A, Karagiannis T, Matthews DR, Tsapas A, et al. Oral semaglutide for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020; 22:335–45.
Article33. Andreadis P, Karagiannis T, Malandris K, Avgerinos I, Liakos A, Manolopoulos A, et al. Semaglutide for type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2018; 20:2255–63.
Article34. ClinicalTrialsgov. Research study comparing new tablets of semaglutide in new doses, in healthy people [Internet]. Bethesda: National Library of Medicine;2020. [cited 2021 Jan 25]. Available from: https://ClinicalTrials.gov/show/NCT04524832 .35. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375:1834–44.
Article36. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019; 381:841–51.
Article37. Novo Nordisk. Conference call on decision to enter phase 3 development in early Alzheimer’s disease and GLP-1 R&D strategy update [Internet]. Bagsvaerd: Novo Nordisk;2020. [cited 2021 Jan 25]. Available from: https://www.novonordisk.com/content/dam/nncorp/global/en/investors/irmaterial/investor_presentations/2020/AD-conference-call-16-December-2020.pdf .38. Kawai T, Sun B, Yoshino H, Feng D, Suzuki Y, Fukazawa M, et al. Structural basis for GLP-1 receptor activation by LY3502970, an orally active nonpeptide agonist. Proc Natl Acad Sci U S A. 2020; 117:29959–67.
Article39. de Graaf C, Donnelly D, Wootten D, Lau J, Sexton PM, Miller LJ, et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: a long march to therapeutic successes. Pharmacol Rev. 2016; 68:954–1013.
Article40. Tibaduiza EC, Chen C, Beinborn M. A small molecule ligand of the glucagon-like peptide 1 receptor targets its amino-terminal hormone binding domain. J Biol Chem. 2001; 276:37787–93.
Article41. Hennen S, Kodra JT, Soroka V, Krogh BO, Wu X, Kaastrup P, et al. Structural insight into antibody-mediated antagonism of the glucagon-like peptide-1 receptor. Sci Rep. 2016; 6:26236.
Article42. Griffith DA, Edmonds DJ, Fortin JP, Kalgutkar AS, Kuzmiski JB, Loria PM, et al. A small molecule oral agonist of the human glucagon-like peptide-1 receptor. bioRxiv. 2020. Sep. 30. https://doi.org/10.1101/2020.09.29.319483 .
Article43. Zhang X, Belousoff MJ, Zhao P, Kooistra AJ, Truong TT, Ang SY, et al. Differential GLP-1R binding and activation by peptide and non-peptide agonists. Mol Cell. 2020; 80:485–500.
Article44. Wang W, Qiao Y, Li Z. New insights into modes of GPCR activation. Trends Pharmacol Sci. 2018; 39:367–86.
Article45. Wootten D, Miller LJ, Koole C, Christopoulos A, Sexton PM. Allostery and biased agonism at class B G protein-coupled receptors. Chem Rev. 2017; 117:111–38.
Article46. Zhao P, Liang YL, Belousoff MJ, Deganutti G, Fletcher MM, Willard FS, et al. Activation of the GLP-1 receptor by a non-peptidic agonist. Nature. 2020; 577:432–6.
Article47. Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018; 6:105–13.
Article48. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015; 373:2247–57.
Article49. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glucagon-like peptide-1 and glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes
- The Global Shortage of Glucagon-Like Peptide-1 Receptor Agonists
- Anti-Obesity Agents on the Horizon
- The Road towards Triple Agonists: Glucagon-Like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide and Glucagon Receptor - An Update
- New Potential Targets of Glucagon-Like Peptide 1 Receptor Agonists in Pancreatic β-Cells and Hepatocytes