Diabetes Metab J.
2020 Oct;44(5):627-639. 10.4093/dmj.2020.0214.
Update on Monogenic Diabetes in Korea
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 2Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2513025
- DOI: http://doi.org/10.4093/dmj.2020.0214
Abstract
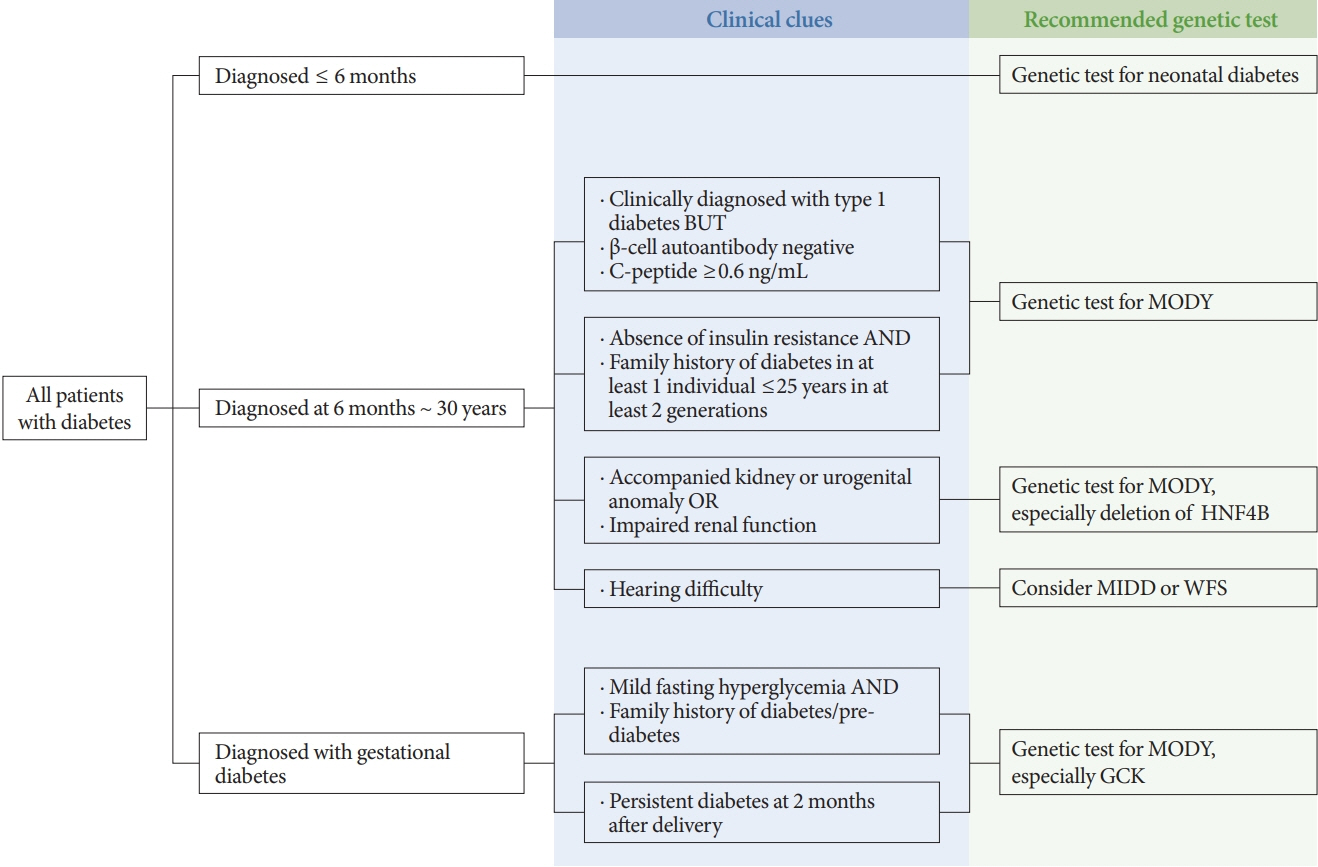
- Monogenic diabetes, including maturity-onset diabetes of the young, neonatal diabetes, and other rare forms of diabetes, results from a single gene mutation. It has been estimated to represent around 1% to 6% of all diabetes. With the advances in genome sequencing technology, it is possible to diagnose more monogenic diabetes cases than ever before. In Korea, 11 studies have identified several monogenic diabetes cases, using Sanger sequencing and whole exome sequencing since 2001. The recent largest study, using targeted exome panel sequencing, found a molecular diagnosis rate of 21.1% for monogenic diabetes in clinically suspected patients. Mutations in glucokinase (GCK), hepatocyte nuclear factor 1α (HNF1A), and HNF4A were most commonly found. Genetic diagnosis of monogenic diabetes is important as it determines the therapeutic approach required for patients and helps to identify affected family members. However, there are still many challenges, which include a lack of simple clinical criterion for selecting patients for genetic testing, difficulties in interpreting the genetic test results, and high costs for genetic testing. In this review, we will discuss the latest updates on monogenic diabetes in Korea, and suggest an algorithm to screen patients for genetic testing. The genetic tests and non-genetic markers for accurate diagnosis of monogenic diabetes will be also reviewed.
Keyword
Figure
Cited by 2 articles
-
Age at Diagnosis and the Risk of Diabetic Nephropathy in Young Patients with Type 1 Diabetes Mellitus (
Diabetes Metab J 2021;45:46-54)
Ye Seul Yang, Tae Seo Sohn
Diabetes Metab J. 2021;45(2):277-278. doi: 10.4093/dmj.2021.0028.Maturity-Onset Diabetes of the Young (MODY)
Seung Shin Park, Soo Heon Kwak
J Korean Diabetes. 2022;23(3):157-164. doi: 10.4093/jkd.2022.23.3.157.
Reference
-
1. Kim SH. Maturity-onset diabetes of the young: what do clinicians need to know? Diabetes Metab J. 2015; 39:468–77.
Article2. Urakami T. Maturity-onset diabetes of the young (MODY): current perspectives on diagnosis and treatment. Diabetes Metab Syndr Obes. 2019; 12:1047–56.3. Tattersall RB. Mild familial diabetes with dominant inheritance. Q J Med. 1974; 43:339–57.4. Panzram G, Adolph W. Heterogeneity of maturity onset diabetes at young age (MODY). Lancet. 1981; 2:986.
Article5. Ledermann HM. Is maturity onset diabetes at young age (MODY) more common in Europe than previously assumed? Lancet. 1995; 345:648.
Article6. Schober E, Rami B, Grabert M, Thon A, Kapellen T, Reinehr T, Holl RW; DPV-Wiss Initiative of the German Working Group for Paediatric Diabetology and. Phenotypical aspects of maturity-onset diabetes of the young (MODY diabetes) in comparison with type 2 diabetes mellitus (T2DM) in children and adolescents: experience from a large multicentre database. Diabet Med. 2009; 26:466–73.
Article7. Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010; 53:2504–8.
Article8. Johansson BB, Irgens HU, Molnes J, Sztromwasser P, Aukrust I, Juliusson PB, Sovik O, Levy S, Skrivarhaug T, Joner G, Molven A, Johansson S, Njolstad PR. Targeted next-generation sequencing reveals MODY in up to 6.5% of antibody-negative diabetes cases listed in the Norwegian Childhood Diabetes Registry. Diabetologia. 2017; 60:625–35.
Article9. Xu JY, Dan QH, Chan V, Wat NM, Tam S, Tiu SC, Lee KF, Siu SC, Tsang MW, Fung LM, Chan KW, Lam KS. Genetic and clinical characteristics of maturity-onset diabetes of the young in Chinese patients. Eur J Hum Genet. 2005; 13:422–7.
Article10. Misra S, Shields B, Colclough K, Johnston DG, Oliver NS, Ellard S, Hattersley AT. South Asian individuals with diabetes who are referred for MODY testing in the UK have a lower mutation pick-up rate than white European people. Diabetologia. 2016; 59:2262–5.
Article11. Kanthimathi S, Jahnavi S, Balamurugan K, Ranjani H, Sonya J, Goswami S, Chowdhury S, Mohan V, Radha V. Glucokinase gene mutations (MODY 2) in Asian Indians. Diabetes Technol Ther. 2014; 16:180–5.
Article12. Li M, Wang S, Xu K, Chen Y, Fu Q, Gu Y, Shi Y, Zhang M, Sun M, Chen H, Han X, Li Y, Tang Z, Cai L, Li Z, Shi Y, Yang T, Polychronakos C. High prevalence of a monogenic cause in Han Chinese diagnosed with type 1 diabetes, partly driven by nonsyndromic recessive WFS1 mutations. Diabetes. 2020; 69:121–6.
Article13. Yorifuji T, Higuchi S, Kawakita R, Hosokawa Y, Aoyama T, Murakami A, Kawae Y, Hatake K, Nagasaka H, Tamagawa N. Genetic basis of early-onset, maturity-onset diabetes of the young-like diabetes in Japan and features of patients without mutations in the major MODY genes: Dominance of maternal inheritance. Pediatr Diabetes. 2018; 19:1164–72.
Article14. Park SS, Jang SS, Ahn CH, Kim JH, Jung HS, Cho YM, Lee YA, Shin CH, Chae JH, Kim JH, Choi SH, Jang HC, Bae JC, Won JC, Kim SH, Kim JI, Kwak SH, Park KS. Identifying pathogenic variants of monogenic diabetes using targeted panel sequencing in an East Asian population. J Clin Endocrinol Metab. 2019; jc.2018-02397.
Article15. Yorifuji T, Fujimaru R, Hosokawa Y, Tamagawa N, Shiozaki M, Aizu K, Jinno K, Maruo Y, Nagasaka H, Tajima T, Kobayashi K, Urakami T. Comprehensive molecular analysis of Japanese patients with pediatric-onset MODY-type diabetes mellitus. Pediatr Diabetes. 2012; 13:26–32.
Article16. Rhee EJ. Diabetes in Asians. Endocrinol Metab (Seoul). 2015; 30:263–9.
Article17. Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010; 375:408–18.
Article18. Lee HJ, Ahn CW, Kim SJ, Song YD, Lim SK, Kim KR, Lee HC, Huh KB. Mutation in hepatocyte nuclear factor-1alpha is not a common cause of MODY and early-onset type 2 diabetes in Korea. Acta Diabetol. 2001; 38:123–7.19. Kim KA, Kang K, Chi YI, Chang I, Lee MK, Kim KW, Shoelson SE, Lee MS. Identification and functional characterization of a novel mutation of hepatocyte nuclear factor-1alpha gene in a Korean family with MODY3. Diabetologia. 2003; 46:721–7.20. Choi IK, Kim DH, Kim HS, Huh N, Paek SH, Jung SY. The prevalence of Maturity Onset Diabetes of the Young (MODY) 3 in children with type 2 diabetes mellitus. Korean J Pediatr. 2004; 47:641–6.21. Hwang JS, Shin CH, Yang SW, Jung SY, Huh N. Genetic and clinical characteristics of Korean maturity-onset diabetes of the young (MODY) patients. Diabetes Res Clin Pract. 2006; 74:75–81.
Article22. Lim DM, Huh N, Park KY. Hepatocyte nuclear factor 1-alpha mutation in normal glucose-tolerant subjects and early-onset type 2 diabetic patients. Korean J Intern Med. 2008; 23:165–9.
Article23. Kim HS, Hwang SH, Choi ES, Park SY, Yim CH, Han KO, Yoon HK, Chung HY, Kim KS, Bok J, Lee JY, Kim SH. Mutation screening of HNF-1alpha gene in Korean women with gestational diabetes mellitus. Korean Diabetes J. 2008; 32:38–43.24. Kim EK, Lee JS, Cheong HI, Chung SS, Kwak SH, Park KS. Identification and functional characterization of P159L mutation in HNF1B in a family with Maturity-Onset Diabetes of the Young 5 (MODY5). Genomics Inform. 2014; 12:240–6.25. Shim YJ, Kim JE, Hwang SK, Choi BS, Choi BH, Cho EM, Jang KM, Ko CW. Identification of candidate gene variants in Korean MODY families by whole-exome sequencing. Horm Res Paediatr. 2015; 83:242–51.
Article26. Kwak SH, Jung CH, Ahn CH, Park J, Chae J, Jung HS, Cho YM, Lee DH, Kim JI, Park KS. Clinical whole exome sequencing in early onset diabetes patients. Diabetes Res Clin Pract. 2016; 122:71–7.
Article27. Cho EH, Min JW, Choi SS, Choi HS, Kim SW. Identification of maturity-onset diabetes of the young caused by glucokinase mutations detected using whole-exome sequencing. Endocrinol Metab (Seoul). 2017; 32:296–301.
Article28. Jang KM. Maturity-onset diabetes of the young: update and perspectives on diagnosis and treatment. Yeungnam Univ J Med. 2020; 37:13–21.
Article29. Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanne-Chantelot C, Ellard S, Gloyn AL. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009; 30:1512–26.
Article30. Steele AM, Wensley KJ, Ellard S, Murphy R, Shepherd M, Colclough K, Hattersley AT, Shields BM. Use of HbA1c in the identification of patients with hyperglycaemia caused by a glucokinase mutation: observational case control studies. PLoS One. 2013; 8:e65326.
Article31. Njolstad PR, Sovik O, Cuesta-Munoz A, Bjorkhaug L, Massa O, Barbetti F, Undlien DE, Shiota C, Magnuson MA, Molven A, Matschinsky FM, Bell GI. Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med. 2001; 344:1588–92.
Article32. Steele AM, Shields BM, Wensley KJ, Colclough K, Ellard S, Hattersley AT. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA. 2014; 311:279–86.
Article33. Fendler W, Rizzo M, Borowiec M, Malachowska B, Antosik K, Szadkowska A, Banach M, Urbanska-Kosinska M, Szopa M, Malecki M, Mlynarski W. Less but better: cardioprotective lipid profile of patients with GCK-MODY despite lower HDL cholesterol level. Acta Diabetol. 2014; 51:625–32.
Article34. Stride A, Shields B, Gill-Carey O, Chakera AJ, Colclough K, Ellard S, Hattersley AT. Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia. 2014; 57:54–6.
Article35. Stanik J, Kusekova M, Huckova M, Valentinova L, Masindova I, Stanikova D, Ferenczova J, Gasperikova D, Klimes I. Impact of type 2 diabetes on glucokinase diabetes (GCK-MODY) phenotype in a Roma (Gypsy) family: case report. Endocr Regul. 2012; 46:99–105.36. Spyer G, Macleod KM, Shepherd M, Ellard S, Hattersley AT. Pregnancy outcome in patients with raised blood glucose due to a heterozygous glucokinase gene mutation. Diabet Med. 2009; 26:14–8.
Article37. Pontoglio M, Sreenan S, Roe M, Pugh W, Ostrega D, Doyen A, Pick AJ, Baldwin A, Velho G, Froguel P, Levisetti M, Bonner-Weir S, Bell GI, Yaniv M, Polonsky KS. Defective insulin secretion in hepatocyte nuclear factor 1alpha-deficient mice. J Clin Invest. 1998; 101:2215–22.
Article38. Yamagata K, Nammo T, Moriwaki M, Ihara A, Iizuka K, Yang Q, Satoh T, Li M, Uenaka R, Okita K, Iwahashi H, Zhu Q, Cao Y, Imagawa A, Tochino Y, Hanafusa T, Miyagawa J, Matsuzawa Y. Overexpression of dominant-negative mutant hepatocyte nuclear fctor-1 alpha in pancreatic beta-cells causes abnormal islet architecture with decreased expression of E-cadherin, reduced beta-cell proliferation, and diabetes. Diabetes. 2002; 51:114–23.39. Valkovicova T, Skopkova M, Stanik J, Gasperikova D. Novel insights into genetics and clinics of the HNF1A-MODY. Endocr Regul. 2019; 53:110–34.
Article40. Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum Mutat. 2013; 34:669–85.
Article41. Shepherd M, Ellis I, Ahmad AM, Todd PJ, Bowen-Jones D, Mannion G, Ellard S, Sparkes AC, Hattersley AT. Predictive genetic testing in maturity-onset diabetes of the young (MODY). Diabet Med. 2001; 18:417–21.
Article42. Steele AM, Shields BM, Shepherd M, Ellard S, Hattersley AT, Pearson ER. Increased all-cause and cardiovascular mortality in monogenic diabetes as a result of mutations in the HNF1A gene. Diabet Med. 2010; 27:157–61.
Article43. Fajans SS, Brown MB. Administration of sulfonylureas can increase glucose-induced insulin secretion for decades in patients with maturity-onset diabetes of the young. Diabetes Care. 1993; 16:1254–61.
Article44. Pearson ER, Liddell WG, Shepherd M, Corrall RJ, Hattersley AT. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1alpha gene mutations: evidence for pharmacogenetics in diabetes. Diabet Med. 2000; 17:543–5.
Article45. Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet. 2003; 362:1275–81.
Article46. Shepherd M, Shields B, Ellard S, Rubio-Cabezas O, Hattersley AT. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med. 2009; 26:437–41.47. Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A. 1997; 94:13209–14.48. Pearson ER, Pruhova S, Tack CJ, Johansen A, Castleden HA, Lumb PJ, Wierzbicki AS, Clark PM, Lebl J, Pedersen O, Ellard S, Hansen T, Hattersley AT. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia. 2005; 48:878–85.49. Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, Ellard S, Ferrer J, Hattersley AT. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007; 4:e118.
Article50. Naylor R, Philipson LH. Who should have genetic testing for maturity-onset diabetes of the young? Clin Endocrinol (Oxf). 2011; 75:422–6.
Article51. Thanabalasingham G, Owen KR. Diagnosis and management of maturity onset diabetes of the young (MODY). BMJ. 2011; 343:d6044.
Article52. Ellard S, Bellanne-Chantelot C, Hattersley AT; European Molecular Genetics Quality Network (EMQN) MODY group. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008; 51:546–53.
Article53. Hattersley AT, Greeley SA, Polak M, Rubio-Cabezas O, Njolstad PR, Mlynarski W, Castano L, Carlsson A, Raile K, Chi DV, Ellard S, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2018: the diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2018; 19 Suppl 27:47–63.
Article54. Shields BM, Shepherd M, Hudson M, McDonald TJ, Colclough K, Peters J, Knight B, Hyde C, Ellard S, Pearson ER, Hattersley AT; UNITED study team. Population-based assessment of a biomarker-based screening pathway to aid diagnosis of monogenic diabetes in young-onset patients. Diabetes Care. 2017; 40:1017–25.
Article55. McDonald TJ, Colclough K, Brown R, Shields B, Shepherd M, Bingley P, Williams A, Hattersley AT, Ellard S. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from Type 1 diabetes. Diabet Med. 2011; 28:1028–33.56. Ludvigsson J, Carlsson A, Forsander G, Ivarsson S, Kockum I, Lernmark A, Lindblad B, Marcus C, Samuelsson U. C-peptide in the classification of diabetes in children and adolescents. Pediatr Diabetes. 2012; 13:45–50.
Article57. Besser RE, Shepherd MH, McDonald TJ, Shields BM, Knight BA, Ellard S, Hattersley AT. Urinary C-peptide creatinine ratio is a practical outpatient tool for identifying hepatocyte nuclear factor 1-{alpha}/hepatocyte nuclear factor 4-{alpha} maturity-onset diabetes of the young from long-duration type 1 diabetes. Diabetes Care. 2011; 34:286–91.58. Owen KR, Stride A, Ellard S, Hattersley AT. Etiological investigation of diabetes in young adults presenting with apparent type 2 diabetes. Diabetes Care. 2003; 26:2088–93.
Article59. Owen KR, Shepherd M, Stride A, Ellard S, Hattersley AT. Heterogeneity in young adult onset diabetes: aetiology alters clinical characteristics. Diabet Med. 2002; 19:758–61.
Article60. Vaxillaire M, Froguel P. Monogenic diabetes in the young, pharmacogenetics and relevance to multifactorial forms of type 2 diabetes. Endocr Rev. 2008; 29:254–64.
Article61. Shields BM, McDonald TJ, Ellard S, Campbell MJ, Hyde C, Hattersley AT. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia. 2012; 55:1265–72.
Article62. Thomas ER, Brackenridge A, Kidd J, Kariyawasam D, Carroll P, Colclough K, Ellard S. Diagnosis of monogenic diabetes: 10-year experience in a large multi-ethnic diabetes center. J Diabetes Investig. 2016; 7:332–7.
Article63. Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noel LH, Velho G, Timsit J. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004; 140:510–7.64. Donovan LE, Severin NE. Maternally inherited diabetes and deafness in a North American kindred: tips for making the diagnosis and review of unique management issues. J Clin Endocrinol Metab. 2006; 91:4737–42.
Article65. Barrett TG, Bundey SE, Macleod AF. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet. 1995; 346:1458–63.
Article66. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–24.
Article67. Owen KR, Skupien J, Malecki MT; CEED3 Consortium. The clinical application of non-genetic biomarkers for differential diagnosis of monogenic diabetes. Diabetes Res Clin Pract. 2009; 86 Suppl 1:S15–21.
Article68. Reiner AP, Barber MJ, Guan Y, Ridker PM, Lange LA, Chasman DI, Walston JD, Cooper GM, Jenny NS, Rieder MJ, Durda JP, Smith JD, Novembre J, Tracy RP, Rotter JI, Stephens M, Nickerson DA, Krauss RM. Polymorphisms of the HNF1A gene encoding hepatocyte nuclear factor-1 alpha are associated with C-reactive protein. Am J Hum Genet. 2008; 82:1193–201.69. Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE, Kwiatkowski D, Cook NR, Miletich JP, Chasman DI. Loci related to metabolic-syndrome pathways including LEPR, HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women’s Genome Health Study. Am J Hum Genet. 2008; 82:1185–92.70. Owen KR, Thanabalasingham G, James TJ, Karpe F, Farmer AJ, McCarthy MI, Gloyn AL. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care. 2010; 33:1919–24.
Article71. McDonald TJ, Shields BM, Lawry J, Owen KR, Gloyn AL, Ellard S, Hattersley AT. High-sensitivity CRP discriminates HNF1A-MODY from other subtypes of diabetes. Diabetes Care. 2011; 34:1860–2.72. Thanabalasingham G, Shah N, Vaxillaire M, Hansen T, Tuomi T, Gasperikova D, Szopa M, Tjora E, James TJ, Kokko P, Loiseleur F, Andersson E, Gaget S, Isomaa B, Nowak N, Raeder H, Stanik J, Njolstad PR, Malecki MT, Klimes I, Groop L, Pedersen O, Froguel P, McCarthy MI, Gloyn AL, Owen KR. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia. 2011; 54:2801–10.
Article73. Juszczak A, Pavic T, Vuckovic F, Bennett AJ, Shah N, Pape Medvidovic E, Groves CJ, Sekerija M, Chandler K, Burrows C, Rojnic Putarek N, Vucic Lovrencic M, Cuca Knezevic J, James TJ, Gloyn AL, Lauc G, McCarthy MI, Owen KR, Gornik O. Plasma fucosylated glycans and C-reactive protein as biomarkers of HNF1A-MODY in young adult-onset nonautoimmune diabetes. Diabetes Care. 2019; 42:17–26.
Article74. Ohki T, Utsu Y, Morita S, Karim MF, Sato Y, Yoshizawa T, Yamamura K, Yamada K, Kasayama S, Yamagata K. Low serum level of high-sensitivity C-reactive protein in a Japanese patient with maturity-onset diabetes of the young type 3 (MODY3). J Diabetes Investig. 2014; 5:513–6.
Article75. Rama Chandran S, Bhalshankar J, Farhad Vasanwala R, Zhao Y, Owen KR, Su-Lyn Gardner D. Traditional clinical criteria outperform high-sensitivity C-reactive protein for the screening of hepatic nuclear factor 1 alpha maturity-onset diabetes of the young among young Asians with diabetes. Ther Adv Endocrinol Metab. 2018; 9:271–82.
Article76. Thanabalasingham G, Huffman JE, Kattla JJ, Novokmet M, Rudan I, Gloyn AL, Hayward C, Adamczyk B, Reynolds RM, Muzinic A, Hassanali N, Pucic M, Bennett AJ, Essafi A, Polasek O, Mughal SA, Redzic I, Primorac D, Zgaga L, Kolcic I, Hansen T, Gasperikova D, Tjora E, Strachan MW, Nielsen T, Stanik J, Klimes I, Pedersen OB, Njølstad PR, Wild SH, Gyllensten U, Gornik O, Wilson JF, Hastie ND, Campbell H, McCarthy MI, Rudd PM, Owen KR, Lauc G, Wright AF. Mutations in HNF1A result in marked alterations of plasma glycan profile. Diabetes. 2013; 62:1329–37.
Article77. Lauc G, Essafi A, Huffman JE, Hayward C, Knezevic A, Kattla JJ, Polasek O, Gornik O, Vitart V, Abrahams JL, Pucic M, Novokmet M, Redzic I, Campbell S, Wild SH, Borovecki F, Wang W, Kolcic I, Zgaga L, Gyllensten U, Wilson JF, Wright AF, Hastie ND, Campbell H, Rudd PM, Rudan I. Genomics meets glycomics-the first GWAS study of human N-Glycome identifies HNF1α as a master regulator of plasma protein fucosylation. PLoS Genet. 2010; 6:e1001256.
Article78. Richter S, Shih DQ, Pearson ER, Wolfrum C, Fajans SS, Hattersley AT, Stoffel M. Regulation of apolipoprotein M gene expression by MODY3 gene hepatocyte nuclear factor-1alpha: haploinsufficiency is associated with reduced serum apolipoprotein M levels. Diabetes. 2003; 52:2989–95.79. Mughal SA, Park R, Nowak N, Gloyn AL, Karpe F, Matile H, Malecki MT, McCarthy MI, Stoffel M, Owen KR. Apolipoprotein M can discriminate HNF1A-MODY from type 1 diabetes. Diabet Med. 2013; 30:246–50.80. Skupien J, Kepka G, Gorczynska-Kosiorz S, Gebska A, Klupa T, Wanic K, Nowak N, Borowiec M, Sieradzki J, Malecki MT. Evaluation of apolipoprotein m serum concentration as a biomarker of HNF-1alpha MODY. Rev Diabet Stud. 2007; 4:231–5.
Article81. Cervin C, Axler O, Holmkvist J, Almgren P, Rantala E, Tuomi T, Groop L, Dahlback B, Karlsson E. An investigation of serum concentration of apoM as a potential MODY3 marker using a novel ELISA. J Intern Med. 2010; 267:316–21.
Article82. Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RH. Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol. 2007; 5:e203.
Article83. Baroukh N, Ravier MA, Loder MK, Hill EV, Bounacer A, Scharfmann R, Rutter GA, Van Obberghen E. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J Biol Chem. 2007; 282:19575–88.84. Poy MN, Spranger M, Stoffel M. microRNAs and the regulation of glucose and lipid metabolism. Diabetes Obes Metab. 2007; 9 Suppl 2:67–73.
Article85. Bonner C, Nyhan KC, Bacon S, Kyithar MP, Schmid J, Concannon CG, Bray IM, Stallings RL, Prehn JH, Byrne MM. Identification of circulating microRNAs in HNF1A-MODY carriers. Diabetologia. 2013; 56:1743–51.
Article86. Bacon S, Engelbrecht B, Schmid J, Pfeiffer S, Gallagher R, McCarthy A, Burke M, Concannon C, Prehn JH, Byrne MM. MicroRNA-224 is readily detectable in urine of individuals with diabetes mellitus and is a potential indicator of beta-cell demise. Genes (Basel). 2015; 6:399–416.87. Zhu Y, You W, Wang H, Li Y, Qiao N, Shi Y, Zhang C, Bleich D, Han X. MicroRNA-24/MODY gene regulatory pathway mediates pancreatic β-cell dysfunction. Diabetes. 2013; 62:3194–206.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monogenic diabetes mellitus and clinical implications of genetic diagnosis
- Monogenic diabetes: recent updates on diagnosis and precision treatment: A narrative review
- Genetic Diseases Associated with Diabetes Mellitus
- Monogenic Thyroid Disorder
- Maturity-Onset Diabetes of the Young: What Do Clinicians Need to Know?