Diabetes Metab J.
2015 Dec;39(6):468-477. 10.4093/dmj.2015.39.6.468.
Maturity-Onset Diabetes of the Young: What Do Clinicians Need to Know?
- Affiliations
-
- 1Division of Endocrinology & Metabolism, Department of Medicine, Cheil General Hospital & Women's Healthcare Center, Dankook University College of Medicine, Seoul, Korea. hoonie.kim@cgh.co.kr
- KMID: 2173998
- DOI: http://doi.org/10.4093/dmj.2015.39.6.468
Abstract
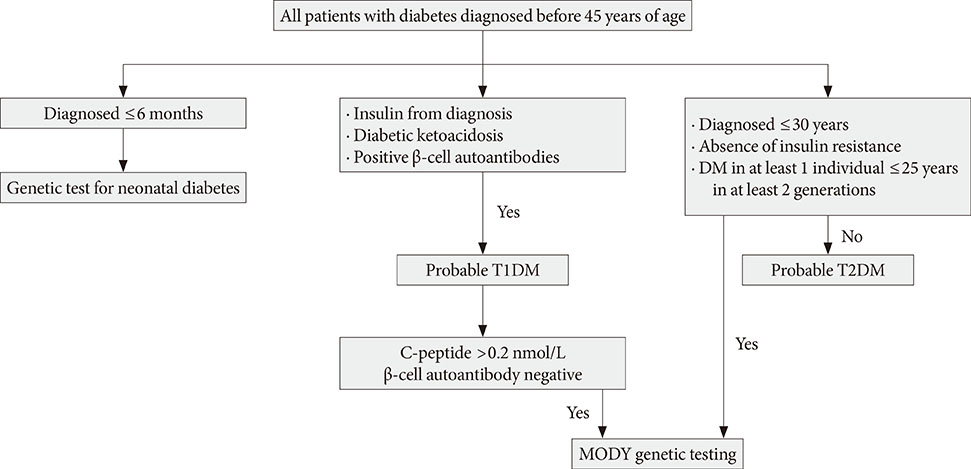
- Maturity-onset diabetes of the young (MODY) is a monogenic form of diabetes that is characterized by an early onset, autosomal dominant mode of inheritance and a primary defect in pancreatic beta-cell function. MODY represents less than 2% of all diabetes cases and is commonly misdiagnosed as type 1 or type 2 diabetes mellitus. At least 13 MODY subtypes with distinct genetic etiologies have been identified to date. A correct genetic diagnosis is important as it often leads to personalized treatment for those with diabetes and enables predictive genetic testing for their asymptomatic relatives. Next-generation sequencing may provide an efficient method for screening mutations in this form of diabetes as well as identifying new MODY genes. In this review, I discuss a current update on MODY in the literatures and cover the studies that have been performed in Korea.
Figure
Cited by 2 articles
-
Update on Monogenic Diabetes in Korea
Ye Seul Yang, Soo Heon Kwak, Kyong Soo Park
Diabetes Metab J. 2020;44(5):627-639. doi: 10.4093/dmj.2020.0214.Maturity-onset diabetes of the young: update and perspectives on diagnosis and treatment
Kyung Mi Jang
Yeungnam Univ J Med. 2020;37(1):13-21. doi: 10.12701/yujm.2019.00409.
Reference
-
1. Tattersall RB. Mild familial diabetes with dominant inheritance. Q J Med. 1974; 43:339–357.2. Ledermann HM. Maturity-onset diabetes of the young (MODY) at least ten times more common in Europe than previously assumed? Diabetologia. 1995; 38:1482.3. Vaxillaire M, Froguel P. Monogenic diabetes in the young, pharmacogenetics and relevance to multifactorial forms of type 2 diabetes. Endocr Rev. 2008; 29:254–264.4. Kavvoura FK, Owen KR. Maturity onset diabetes of the young: clinical characteristics, diagnosis and management. Pediatr Endocrinol Rev. 2012; 10:234–242.5. Hwang JS, Shin CH, Yang SW, Jung SY, Huh N. Genetic and clinical characteristics of Korean maturity-onset diabetes of the young (MODY) patients. Diabetes Res Clin Pract. 2006; 74:75–81.6. Hwang JS. MODY syndrome. J Korean Soc Pediatr Endocrinol. 2010; 15:1–6.7. Nishigori H, Yamada S, Kohama T, Utsugi T, Shimizu H, Takeuchi T, Takeda J. Mutations in the hepatocyte nuclear factor-1 alpha gene (MODY3) are not a major cause of early-onset non-insulin-dependent (type 2) diabetes mellitus in Japanese. J Hum Genet. 1998; 43:107–110.8. Xu JY, Dan QH, Chan V, Wat NM, Tam S, Tiu SC, Lee KF, Siu SC, Tsang MW, Fung LM, Chan KW, Lam KS. Genetic and clinical characteristics of maturity-onset diabetes of the young in Chinese patients. Eur J Hum Genet. 2005; 13:422–427.9. Froguel P, Vaxillaire M, Sun F, Velho G, Zouali H, Butel MO, Lesage S, Vionnet N, Clement K, Fougerousse F, Tanizawa Y, Weissenbach J, Beckmann JS, Lathrop GM, Passa PH, Permutt MA, Cohen D. Close linkage of glucokinase locus on chromosome 7p to early-onset non-insulin-dependent diabetes mellitus. Nature. 1992; 356:162–164.10. Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanne-Chantelot C, Ellard S, Gloyn AL. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009; 30:1512–1526.11. Yorifuji T, Fujimaru R, Hosokawa Y, Tamagawa N, Shiozaki M, Aizu K, Jinno K, Maruo Y, Nagasaka H, Tajima T, Kobayashi K, Urakami T. Comprehensive molecular analysis of Japanese patients with pediatric-onset MODY-type diabetes mellitus. Pediatr Diabetes. 2012; 13:26–32.12. Feigerlova E, Pruhova S, Dittertova L, Lebl J, Pinterova D, Kolostova K, Cerna M, Pedersen O, Hansen T. Aetiological heterogeneity of asymptomatic hyperglycaemia in children and adolescents. Eur J Pediatr. 2006; 165:446–452.13. Steele AM, Shields BM, Wensley KJ, Colclough K, Ellard S, Hattersley AT. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA. 2014; 311:279–286.14. Kawakita R, Hosokawa Y, Fujimaru R, Tamagawa N, Urakami T, Takasawa K, Moriya K, Mizuno H, Maruo Y, Takuwa M, Nagasaka H, Nishi Y, Yamamoto Y, Aizu K, Yorifuji T. Molecular and clinical characterization of glucokinase maturity-onset diabetes of the young (GCK-MODY) in Japanese patients. Diabet Med. 2014; 31:1357–1362.15. Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue KC. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2009; 10:Suppl 12. 33–42.16. Spyer G, Macleod KM, Shepherd M, Ellard S, Hattersley AT. Pregnancy outcome in patients with raised blood glucose due to a heterozygous glucokinase gene mutation. Diabet Med. 2009; 26:14–18.17. Dukes ID, Sreenan S, Roe MW, Levisetti M, Zhou YP, Ostrega D, Bell GI, Pontoglio M, Yaniv M, Philipson L, Polonsky KS. Defective pancreatic beta-cell glycolytic signaling in hepatocyte nuclear factor-1alpha-deficient mice. J Biol Chem. 1998; 273:24457–24464.18. Hattersley AT. Maturity-onset diabetes of the young: clinical heterogeneity explained by genetic heterogeneity. Diabet Med. 1998; 15:15–24.19. Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum Mutat. 2013; 34:669–685.20. Kim KA, Kang K, Chi YI, Chang I, Lee MK, Kim KW, Shoelson SE, Lee MS. Identification and functional characterization of a novel mutation of hepatocyte nuclear factor-1alpha gene in a Korean family with MODY3. Diabetologia. 2003; 46:721–727.21. Harries LW, Ellard S, Stride A, Morgan NG, Hattersley AT. Isomers of the TCF1 gene encoding hepatocyte nuclear factor-1 alpha show differential expression in the pancreas and define the relationship between mutation position and clinical phenotype in monogenic diabetes. Hum Mol Genet. 2006; 15:2216–2224.22. Pontoglio M, Prie D, Cheret C, Doyen A, Leroy C, Froguel P, Velho G, Yaniv M, Friedlander G. HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep. 2000; 1:359–365.23. Steele AM, Shields BM, Shepherd M, Ellard S, Hattersley AT, Pearson ER. Increased all-cause and cardiovascular mortality in monogenic diabetes as a result of mutations in the HNF1A gene. Diabet Med. 2010; 27:157–161.24. Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet. 2003; 362:1275–1281.25. Shepherd M, Shields B, Ellard S, Rubio-Cabezas O, Hattersley AT. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med. 2009; 26:437–441.26. Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A. 1997; 94:13209–13214.27. Pearson ER, Pruhova S, Tack CJ, Johansen A, Castleden HA, Lumb PJ, Wierzbicki AS, Clark PM, Lebl J, Pedersen O, Ellard S, Hansen T, Hattersley AT. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia. 2005; 48:878–885.28. Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, Ellard S, Ferrer J, Hattersley AT. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007; 4:e118.29. Lehto M, Bitzen PO, Isomaa B, Wipemo C, Wessman Y, Forsblom C, Tuomi T, Taskinen MR, Groop L. Mutation in the HNF-4alpha gene affects insulin secretion and triglyceride metabolism. Diabetes. 1999; 48:423–425.30. Stoffers DA, Thomas MK, Habener JF. Homeodomain protein IDX-1: a master regulator of pancreas development and insulin gene expression. Trends Endocrinol Metab. 1997; 8:145–151.31. Schwitzgebel VM, Mamin A, Brun T, Ritz-Laser B, Zaiko M, Maret A, Jornayvaz FR, Theintz GE, Michielin O, Melloul D, Philippe J. Agenesis of human pancreas due to decreased half-life of insulin promoter factor 1. J Clin Endocrinol Metab. 2003; 88:4398–4406.32. Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997; 17:138–139.33. Coffinier C, Thepot D, Babinet C, Yaniv M, Barra J. Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999; 126:4785–4794.34. Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet. 1997; 17:384–385.35. Edghill EL, Bingham C, Slingerland AS, Minton JA, Noordam C, Ellard S, Hattersley AT. Hepatocyte nuclear factor-1 beta mutations cause neonatal diabetes and intrauterine growth retardation: support for a critical role of HNF-1beta in human pancreatic development. Diabet Med. 2006; 23:1301–1306.36. Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noel LH, Velho G, Timsit J. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004; 140:510–517.37. Chen YZ, Gao Q, Zhao XZ, Chen YZ, Bennett CL, Xiong XS, Mei CL, Shi YQ, Chen XM. Systematic review of TCF2 anomalies in renal cysts and diabetes syndrome/maturity onset diabetes of the young type 5. Chin Med J (Engl). 2010; 123:3326–3333.38. Kim EK, Lee JS, Cheong HI, Chung SS, Kwak SH, Park KS. Identification and functional characterization of P159L mutation in HNF1B in a family with maturity-onset diabetes of the young 5 (MODY5). Genomics Inform. 2014; 12:240–246.39. Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, Clauin S, Deschenes G, Bouissou F, Bensman A, Bellanne-Chantelot C. Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol. 2006; 17:497–503.40. Pearson ER, Badman MK, Lockwood CR, Clark PM, Ellard S, Bingham C, Hattersley AT. Contrasting diabetes phenotypes associated with hepatocyte nuclear factor-1alpha and -1beta mutations. Diabetes Care. 2004; 27:1102–1107.41. Malecki MT, Jhala US, Antonellis A, Fields L, Doria A, Orban T, Saad M, Warram JH, Montminy M, Krolewski AS. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet. 1999; 23:323–328.42. Gonsorcikova L, Pruhova S, Cinek O, Ek J, Pelikanova T, Jorgensen T, Eiberg H, Pedersen O, Hansen T, Lebl J. Autosomal inheritance of diabetes in two families characterized by obesity and a novel H241Q mutation in NEUROD1. Pediatr Diabetes. 2008; 9(4 Pt 2):367–372.43. Rubio-Cabezas O, Minton JA, Kantor I, Williams D, Ellard S, Hattersley AT. Homozygous mutations in NEUROD1 are responsible for a novel syndrome of permanent neonatal diabetes and neurological abnormalities. Diabetes. 2010; 59:2326–2331.44. Fernandez-Zapico ME, van Velkinburgh JC, Gutierrez-Aguilar R, Neve B, Froguel P, Urrutia R, Stein R. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet beta cells. J Biol Chem. 2009; 284:36482–36490.45. Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, Dina C, Hamid YH, Joly E, Vaillant E, Benmezroua Y, Durand E, Bakaher N, Delannoy V, Vaxillaire M, Cook T, Dallinga-Thie GM, Jansen H, Charles MA, Clement K, Galan P, Hercberg S, Helbecque N, Charpentier G, Prentki M, Hansen T, Pedersen O, Urrutia R, Melloul D, Froguel P. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005; 102:4807–4812.46. Raeder H, Johansson S, Holm PI, Haldorsen IS, Mas E, Sbarra V, Nermoen I, Eide SA, Grevle L, Bjorkhaug L, Sagen JV, Aksnes L, Sovik O, Lombardo D, Molven A, Njolstad PR. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet. 2006; 38:54–62.47. Johansson BB, Torsvik J, Bjorkhaug L, Vesterhus M, Ragvin A, Tjora E, Fjeld K, Hoem D, Johansson S, Raeder H, Lindquist S, Hernell O, Cnop M, Saraste J, Flatmark T, Molven A, Njolstad PR. Diabetes and pancreatic exocrine dysfunction due to mutations in the carboxyl ester lipase gene-maturity onset diabetes of the young (CEL-MODY): a protein misfolding disease. J Biol Chem. 2011; 286:34593–34605.48. Plengvidhya N, Kooptiwut S, Songtawee N, Doi A, Furuta H, Nishi M, Nanjo K, Tantibhedhyangkul W, Boonyasrisawat W, Yenchitsomanus PT, Doria A, Banchuin N. PAX4 mutations in Thais with maturity onset diabetes of the young. J Clin Endocrinol Metab. 2007; 92:2821–2826.49. Mauvais-Jarvis F, Smith SB, Le May C, Leal SM, Gautier JF, Molokhia M, Riveline JP, Rajan AS, Kevorkian JP, Zhang S, Vexiau P, German MS, Vaisse C. PAX4 gene variations predispose to ketosis-prone diabetes. Hum Mol Genet. 2004; 13:3151–3159.50. Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Stoy J, Steiner DF, Philipson LH, Bell GI, Hattersley AT, Ellard S. Neonatal Diabetes International Collaborative Group. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008; 57:1034–1042.51. Meur G, Simon A, Harun N, Virally M, Dechaume A, Bonnefond A, Fetita S, Tarasov AI, Guillausseau PJ, Boesgaard TW, Pedersen O, Hansen T, Polak M, Gautier JF, Froguel P, Rutter GA, Vaxillaire M. Insulin gene mutations resulting in early-onset diabetes: marked differences in clinical presentation, metabolic status, and pathogenic effect through endoplasmic reticulum retention. Diabetes. 2010; 59:653–661.52. Borowiec M, Liew CW, Thompson R, Boonyasrisawat W, Hu J, Mlynarski WM, El Khattabi I, Kim SH, Marselli L, Rich SS, Krolewski AS, Bonner-Weir S, Sharma A, Sale M, Mychaleckyj JC, Kulkarni RN, Doria A. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc Natl Acad Sci U S A. 2009; 106:14460–14465.53. Kim SH, Ma X, Weremowicz S, Ercolino T, Powers C, Mlynarski W, Bashan KA, Warram JH, Mychaleckyj J, Rich SS, Krolewski AS, Doria A. Identification of a locus for maturity-onset diabetes of the young on chromosome 8p23. Diabetes. 2004; 53:1375–1384.54. Bowman P, Flanagan SE, Edghill EL, Damhuis A, Shepherd MH, Paisey R, Hattersley AT, Ellard S. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia. 2012; 55:123–127.55. Bonnefond A, Philippe J, Durand E, Dechaume A, Huyvaert M, Montagne L, Marre M, Balkau B, Fajardy I, Vambergue A, Vatin V, Delplanque J, Le Guilcher D, De Graeve F, Lecoeur C, Sand O, Vaxillaire M, Froguel P. Whole-exome sequencing and high throughput genotyping identified KCNJ11 as the thirteenth MODY gene. PLoS One. 2012; 7:e37423.56. Liu L, Nagashima K, Yasuda T, Liu Y, Hu HR, He G, Feng B, Zhao M, Zhuang L, Zheng T, Friedman TC, Xiang K. Mutations in KCNJ11 are associated with the development of autosomal dominant, early-onset type 2 diabetes. Diabetologia. 2013; 56:2609–2618.57. Shim YJ, Kim JE, Hwang SK, Choi BS, Choi BH, Cho EM, Jang KM, Ko CW. Identification of candidate gene variants in Korean MODY families by whole-exome sequencing. Horm Res Paediatr. 2015; 83:242–251.58. Lee HJ, Ahn CW, Kim SJ, Song YD, Lim SK, Kim KR, Lee HC, Huh KB. Mutation in hepatocyte nuclear factor-1alpha is not a common cause of MODY and early-onset type 2 diabetes in Korea. Acta Diabetol. 2001; 38:123–127.59. Choi IK, Kim DH, Kim HS, Huh N, Paek SH, Jung SY. The prevalence of maturity onset diabetes of the young (MODY) 3 in children with type 2 diabetes mellitus. Korean J Pediatr. 2004; 47:641–646.60. Lim DM, Huh N, Park KY. Hepatocyte nuclear factor 1-alpha mutation in normal glucose-tolerant subjects and early-onset type 2 diabetic patients. Korean J Intern Med. 2008; 23:165–169.61. Kim HS, Hwang SH, Choi ES, Park SY, Yim CH, Han KO, Yoon HK, Chung HY, Kim KS, Bok J, Lee JY, Kim SH. Mutation screening of HNF-1alpha gene in Korean women with gestational diabetes mellitus. Korean Diabetes J. 2008; 32:38–43.62. Ellard S, Lango Allen H, De Franco E, Flanagan SE, Hysenaj G, Colclough K, Houghton JA, Shepherd M, Hattersley AT, Weedon MN, Caswell R. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013; 56:1958–1963.63. Johansson S, Irgens H, Chudasama KK, Molnes J, Aerts J, Roque FS, Jonassen I, Levy S, Lima K, Knappskog PM, Bell GI, Molven A, Njolstad PR. Exome sequencing and genetic testing for MODY. PLoS One. 2012; 7:e38050.64. Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010; 53:2504–2508.65. Thanabalasingham G, Pal A, Selwood MP, Dudley C, Fisher K, Bingley PJ, Ellard S, Farmer AJ, McCarthy MI, Owen KR. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012; 35:1206–1212.66. Shields BM, McDonald TJ, Ellard S, Campbell MJ, Hyde C, Hattersley AT. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia. 2012; 55:1265–1272.67. Owen KR, Thanabalasingham G, James TJ, Karpe F, Farmer AJ, McCarthy MI, Gloyn AL. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care. 2010; 33:1919–1924.68. McDonald TJ, Shields BM, Lawry J, Owen KR, Gloyn AL, Ellard S, Hattersley AT. High-sensitivity CRP discriminates HNF1A-MODY from other subtypes of diabetes. Diabetes Care. 2011; 34:1860–1862.69. Thanabalasingham G, Shah N, Vaxillaire M, Hansen T, Tuomi T, Gasperikova D, Szopa M, Tjora E, James TJ, Kokko P, Loiseleur F, Andersson E, Gaget S, Isomaa B, Nowak N, Raeder H, Stanik J, Njolstad PR, Malecki MT, Klimes I, Groop L, Pedersen O, Froguel P, McCarthy MI, Gloyn AL, Owen KR. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia. 2011; 54:2801–2810.70. Bonner C, Nyhan KC, Bacon S, Kyithar MP, Schmid J, Concannon CG, Bray IM, Stallings RL, Prehn JH, Byrne MM. Identification of circulating microRNAs in HNF1A-MODY carriers. Diabetologia. 2013; 56:1743–1751.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Maturity-onset diabetes of the young: update and perspectives on diagnosis and treatment
- A rare, likely pathogenic GCK variant related to maturity-onset diabetes of the young type 2: A case report
- MODY Syndrome
- Identification of Maturity-Onset Diabetes of the Young Caused by Glucokinase Mutations Detected Using Whole-Exome Sequencing
- Monogenic diabetes: recent updates on diagnosis and precision treatment: A narrative review