Korean J Physiol Pharmacol.
2021 Mar;25(2):167-175. 10.4196/kjpp.2021.25.2.167.
Far-infrared rays enhance mitochondrial biogenesis and GLUT3 expression under low glucose conditions in rat skeletal muscle cells
- Affiliations
-
- 1Departments of Physiology, College of Medicine, Chung-Ang University, Seoul 06974, Korea,
- 2Departments of Medicine, College of Medicine, Chung-Ang University, Seoul 06974, Korea,
- 3Cardiovascular and Metabolic Disease Center, SMART Marine Therapeutics Center, Inje University, Busan 47392, Korea,
- 4Department of Family Medicine, College of Medicine, Chung-Ang University Hospital, Seoul 06973, Korea,
- 5Department of Physiology and Cell Biology, University of Nevada, Reno School of Medicine, Reno, NV 89557, USA
- KMID: 2512960
- DOI: http://doi.org/10.4196/kjpp.2021.25.2.167
Abstract
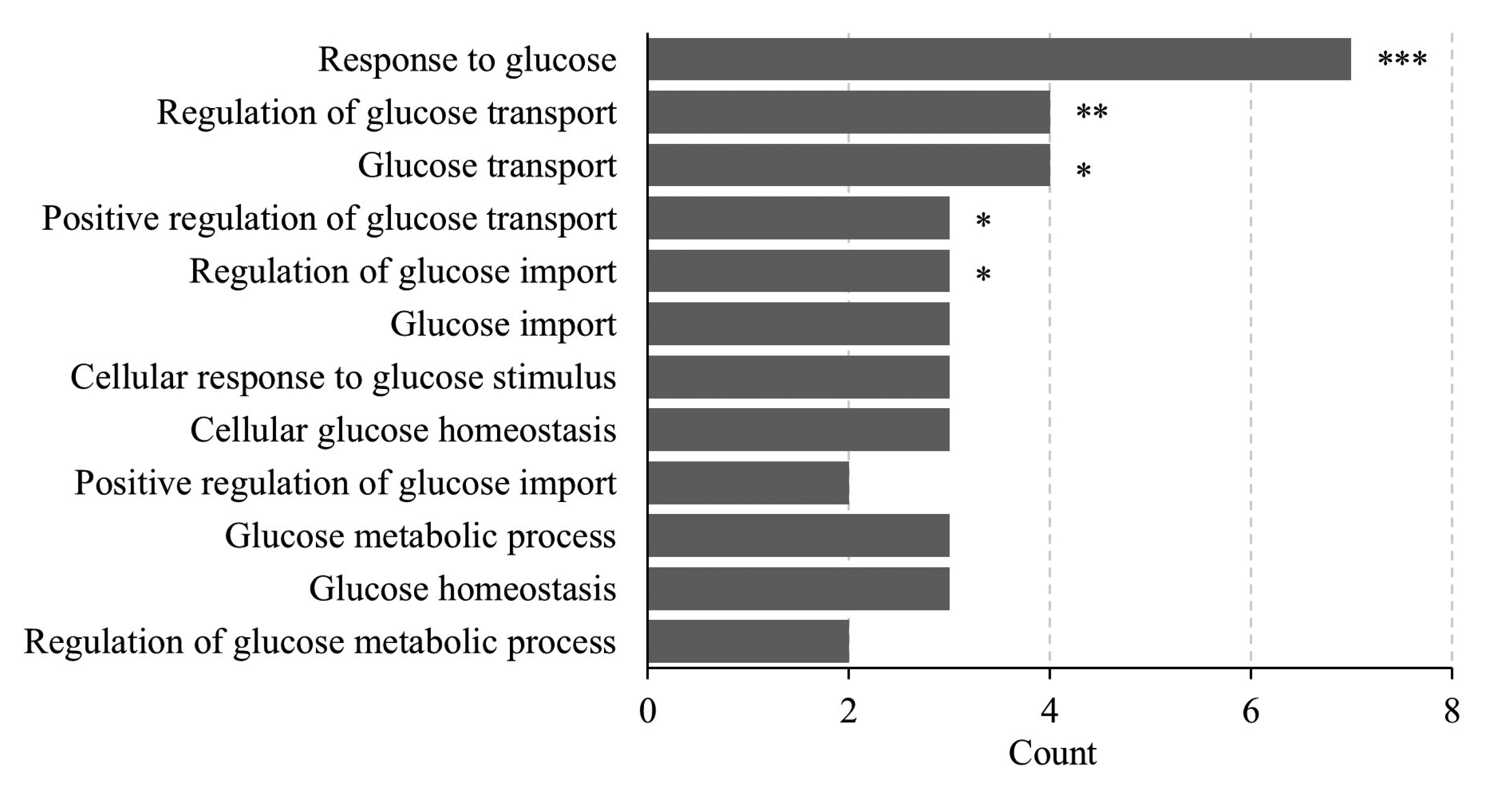
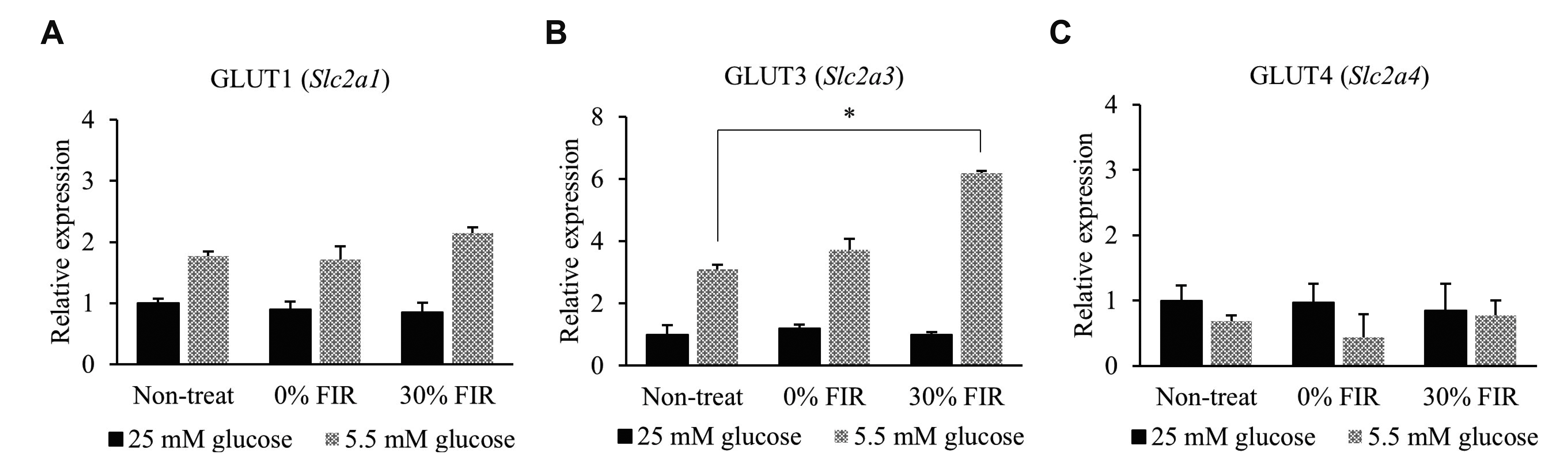
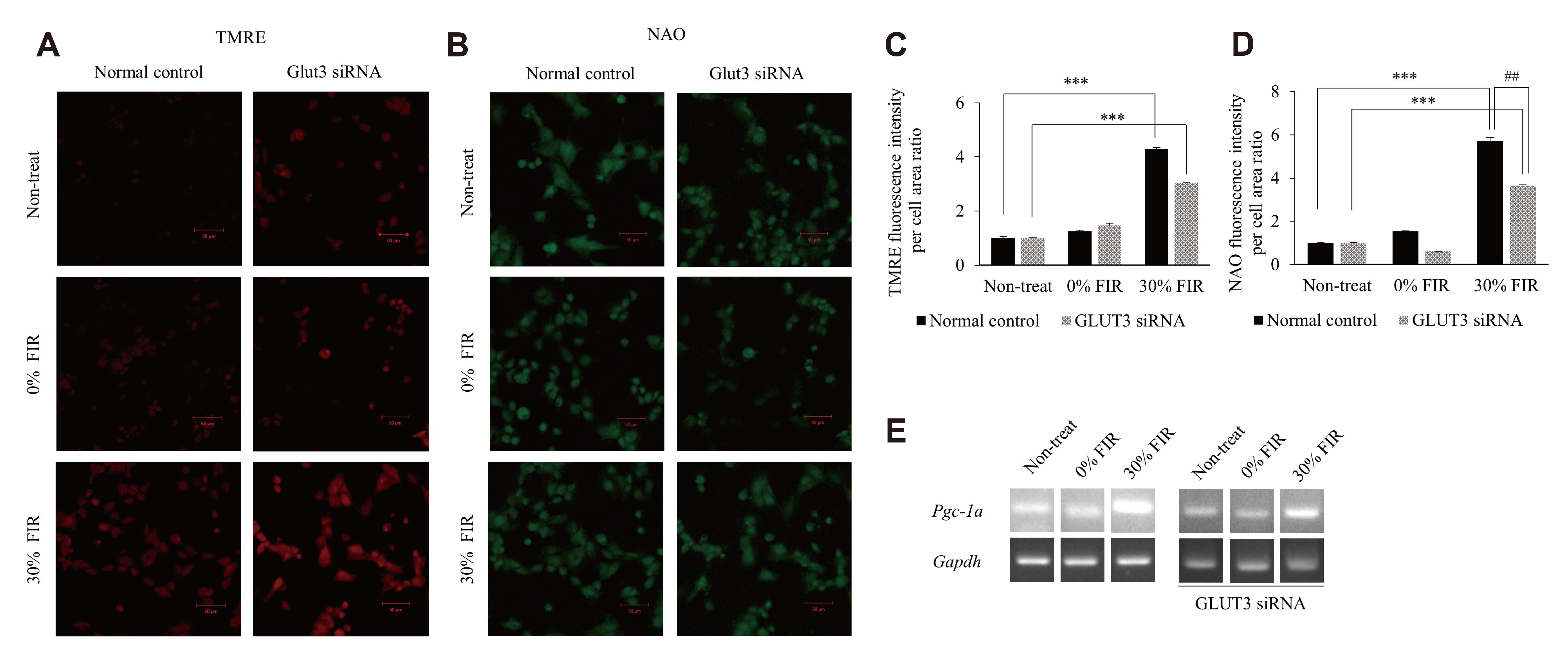
- Far-infrared rays (FIR) are known to have various effects on atoms and molecular structures within cells owing to their radiation and vibration frequencies. The present study examined the effects of FIR on gene expression related to glucose transport through microarray analysis in rat skeletal muscle cells, as well as on mitochondrial biogenesis, at high and low glucose conditions. FIR were emitted from a bio-active material coated fabric (BMCF). L6 cells were treated with 30% BMCF for 24 h in medium containing 25 or 5.5 mM glucose, and changes in the expression of glucose transporter genes were determined. The expression of GLUT3 (Slc2a3) increased 2.0-fold (p < 0.05) under 5.5 mM glucose and 30% BMCF. In addition, mitochondrial oxygen consumption and membrane potential (ΔΨm) increased 1.5- and 3.4-fold (p < 0.05 and p < 0.001), respectively, but no significant change in expression of Pgc-1a, a regulator of mitochondrial biogenesis, was observed in 24 h. To analyze the relationship between GLUT3 expression and mitochondrial biogenesis under FIR, GLUT3 was down-modulated by siRNA for 72 h. As a result, the ΔΨm of the GLUT3 siRNA-treated cells increased 3.0-fold (p < 0.001), whereas that of the control group increased 4.6-fold (p < 0.001). Moreover, Pgc-1a expression increased upon 30% BMCF treatment for 72 h; an effect that was more pronounced in the presence of GLUT3. These results suggest that FIR may hold therapeutic potential for improving glucose metabolism and mitochondrial function in metabolic diseases associated with insufficient glucose supply, such as type 2 diabetes.
Figure
Reference
-
1. Vatansever F, Hamblin MR. 2012; Far infrared radiation (FIR): its biological effects and medical applications. Photonics Lasers Med. 4:255–266. DOI: 10.1515/plm-2012-0034. PMID: 23833705. PMCID: PMC3699878.
Article2. Inoué S, Kabaya M. 1989; Biological activities caused by far-infrared radiation. Int J Biometeorol. 33:145–150. DOI: 10.1007/BF01084598. PMID: 2689357.
Article3. Rau CS, Yang JC, Jeng SF, Chen YC, Lin CJ, Wu CJ, Lu TH, Hsieh CH. 2011; Far-infrared radiation promotes angiogenesis in human microvascular endothelial cells via extracellular signal-regulated kinase activation. Photochem Photobiol. 87:441–446. DOI: 10.1111/j.1751-1097.2010.00853.x. PMID: 21143604.4. Lee D, Kim YW, Kim JH, Yang M, Bae H, Lim I, Bang H, Go KC, Yang GW, Rho YH, Park HS, Park EH, Ko JH. 2015; Improvement characteristics of bio-active materials coated fabric on rat muscular mitochondria. Korean J Physiol Pharmacol. 19:283–289. DOI: 10.4196/kjpp.2015.19.3.283. PMID: 25954135. PMCID: PMC4422970.
Article5. Brunmair B, Staniek K, Dörig J, Szöcs Z, Stadlbauer K, Marian V, Gras F, Anderwald C, Nohl H, Waldhäusl W, Fürnsinn C. 2006; Activation of PPAR-delta in isolated rat skeletal muscle switches fuel preference from glucose to fatty acids. Diabetologia. 49:2713–2722. DOI: 10.1007/s00125-006-0357-6. PMID: 16960684.6. Reddy PH. 2009; Role of mitochondria in neurodegenerative diseases: mitochondria as a therapeutic target in Alzheimer's disease. CNS Spectr. 14(8 Suppl 7):8–13. discussion 16–18. DOI: 10.1017/S1092852900024901. PMID: 19890241. PMCID: PMC3056539.
Article7. Kirkinezos IG, Moraes CT. 2001; Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol. 12:449–457. DOI: 10.1006/scdb.2001.0282. PMID: 11735379.
Article8. Wallace DC. 2007; Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu Rev Biochem. 76:781–821. DOI: 10.1146/annurev.biochem.76.081205.150955. PMID: 17506638.
Article9. Rocha M, Apostolova N, Diaz-Rua R, Muntane J, Victor VM. 2020; Mitochondria and T2D: role of autophagy, ER stress, and inflammasome. Trends Endocrinol Metab. 31:725–741. DOI: 10.1016/j.tem.2020.03.004. PMID: 32265079.
Article10. Frantz MC, Wipf P. 2010; Mitochondria as a target in treatment. Environ Mol Mutagen. 51:462–475. DOI: 10.1002/em.20554. PMID: 20175113. PMCID: PMC2920596.
Article11. Moraes CT, Bacman SR, Williams SL. 2014; Manipulating mitochondrial genomes in the clinic: playing by different rules. Trends Cell Biol. 24:209–211. DOI: 10.1016/j.tcb.2014.02.002. PMID: 24679453. PMCID: PMC4274037.
Article12. Carruthers A. 1990; Facilitated diffusion of glucose. Physiol Rev. 70:1135–1176. DOI: 10.1152/physrev.1990.70.4.1135. PMID: 2217557.
Article13. Khayat ZA, McCall AL, Klip A. 1998; Unique mechanism of GLUT3 glucose transporter regulation by prolonged energy demand: increased protein half-life. Biochem J. 333(Pt 3):713–718. DOI: 10.1042/bj3330713. PMID: 9677332. PMCID: PMC1219636.
Article14. Weijers RNM. 2014; Membrane flexibility and cellular energy management in type 2 diabetes, gestational diabetes, and obesity. EMJ Diabet. 2:65–72.15. Ancey PB, Contat C, Meylan E. 2018; Glucose transporters in cancer - from tumor cells to the tumor microenvironment. FEBS J. 285:2926–2943. DOI: 10.1111/febs.14577. PMID: 29893496.
Article16. Bilan PJ, Mitsumoto Y, Maher F, Simpson IA, Klip A. 1992; Detection of the GLUT3 facilitative glucose transporter in rat L6 muscle cells: regulation by cellular differentiation, insulin and insulin-like growth factor-I. Biochem Biophys Res Commun. 186:1129–1137. DOI: 10.1016/0006-291X(92)90864-H. PMID: 1497646.
Article17. Yaffe D. 1968; Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 61:477–483. DOI: 10.1073/pnas.61.2.477. PMID: 5245982. PMCID: PMC225183.
Article18. Klip A, Li G, Logan WJ. 1984; Induction of sugar uptake response to insulin by serum depletion in fusing L6 myoblasts. Am J Physiol. 247(3 Pt 1):E291–E296. DOI: 10.1152/ajpendo.1984.247.3.E291. PMID: 6383069.
Article19. Ziel FH, Venkatesan N, Davidson MB. 1988; Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 37:885–890. DOI: 10.2337/diab.37.7.885. PMID: 3290006.
Article20. Furler SM, Jenkins AB, Storlien LH, Kraegen EW. 1991; In vivo location of the rate-limiting step of hexose uptake in muscle and brain tissue of rats. Am J Physiol. 261(3 Pt 1):E337–E347. DOI: 10.1152/ajpendo.1991.261.3.E337. PMID: 1887881.
Article21. Webb GC, Akbar MS, Zhao C, Steiner DF. 2000; Expression profiling of pancreatic beta cells: glucose regulation of secretory and metabolic pathway genes. Proc Natl Acad Sci U S A. 97:5773–5778. DOI: 10.1073/pnas.100126597. PMID: 10811900. PMCID: PMC18509.
Article22. Kichenbrand C, Grossin L, Menu P, Moby V. 2020; Behaviour of human dental pulp stem cell in high glucose condition: impact on proliferation and osteogenic differentiation. Arch Oral Biol. 118:104859. DOI: 10.1016/j.archoralbio.2020.104859. PMID: 32768712.
Article23. Lee D, Seo Y, Kim YW, Kim S, Bae H, Choi J, Lim I, Bang H, Kim JH, Ko JH. 2019; Far-infrared radiation stimulates platelet-derived growth factor mediated skeletal muscle cell migration through extracellular matrix-integrin signaling. Korean J Physiol Pharmacol. 23:141–150. DOI: 10.4196/kjpp.2019.23.2.141. PMID: 30820158. PMCID: PMC6384197.
Article24. Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. DOI: 10.1006/meth.2001.1262. PMID: 11846609.25. Frezza C, Cipolat S, Scorrano L. 2007; Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2:287–295. DOI: 10.1038/nprot.2006.478. PMID: 17406588.26. Zhao RZ, Jiang S, Zhang L, Yu ZB. 2019; Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med. 44:3–15. DOI: 10.3892/ijmm.2019.4188. PMID: 31115493. PMCID: PMC6559295.
Article27. Scaduto RC Jr, Grotyohann LW. 1999; Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 76(1 Pt 1):469–477. DOI: 10.1016/S0006-3495(99)77214-0. PMID: 9876159. PMCID: PMC1302536.
Article28. Jeong SH, Kim HK, Song IS, Noh SJ, Marquez J, Ko KS, Rhee BD, Kim N, Mishchenko NP, Fedoreyev SA, Stonik VA, Han J. 2014; Echinochrome a increases mitochondrial mass and function by modulating mitochondrial biogenesis regulatory genes. Mar Drugs. 12:4602–4615. DOI: 10.3390/md12084602. PMID: 25196935. PMCID: PMC4145333.
Article29. Roels J, Vernaillen F, Kremer A, Gonçalves A, Aelterman J, Luong HQ, Goossens B, Philips W, Lippens S, Saeys Y. 2020; An interactive ImageJ plugin for semi-automated image denoising in electron microscopy. Nat Commun. 11:771. DOI: 10.1038/s41467-020-14529-0. PMID: 32034132. PMCID: PMC7005902.
Article30. Weisová P, Concannon CG, Devocelle M, Prehn JH, Ward MW. 2009; Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 29:2997–3008. DOI: 10.1523/JNEUROSCI.0354-09.2009. PMID: 19261894. PMCID: PMC6666202.31. Navale AM, Paranjape AN. 2016; Glucose transporters: physiological and pathological roles. Biophys Rev. 8:5–9. DOI: 10.1007/s12551-015-0186-2. PMID: 28510148. PMCID: PMC5425736.
Article32. Evans PL, McMillin SL, Weyrauch LA, Witczak CA. 2019; Regulation of skeletal muscle glucose transport and glucose metabolism by exercise training. Nutrients. 11:2432. DOI: 10.3390/nu11102432. PMID: 31614762. PMCID: PMC6835691.
Article33. Zhou B, Tian R. 2018; Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 128:3716–3726. DOI: 10.1172/JCI120849. PMID: 30124471. PMCID: PMC6118589.
Article34. Maher F, Vannucci SJ, Simpson IA. 1994; Glucose transporter proteins in brain. FASEB J. 8:1003–1011. DOI: 10.1096/fasebj.8.13.7926364. PMID: 7926364.
Article35. Guillet-Deniau I, Leturque A, Girard J. 1994; Expression and cellular localization of glucose transporters (GLUT1, GLUT3, GLUT4) during differentiation of myogenic cells isolated from rat foetuses. J Cell Sci. 107(Pt 3):487–496. PMID: 8006068.
Article36. Tsakiridis T, Vranic M, Klip A. 1995; Phosphatidylinositol 3-kinase and the actin network are not required for the stimulation of glucose transport caused by mitochondrial uncoupling: comparison with insulin action. Biochem J. 309(Pt 1):1–5. DOI: 10.1042/bj3090001. PMID: 7619042. PMCID: PMC1135791.
Article37. Stuart CA, Wen G, Thompson EA. 1999; Abnormal expression and distribution of glucose transporters in muscle of insulin resistant subjects. Diabetes. 48:SA262.38. Clay Montier LL, Deng JJ, Bai Y. 2009; Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics. 36:125–131. DOI: 10.1016/S1673-8527(08)60099-5. PMID: 19302968. PMCID: PMC4706993.
Article39. Nicholls DG. 2002; Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol. 34:1372–1381. DOI: 10.1016/S1357-2725(02)00077-8. PMID: 12200032.
Article40. Walczak J, Malińska D, Drabik K, Michalska B, Prill M, Johne S, Luettich K, Szymański J, Peitsch MC, Hoeng J, Duszyński J, Więckowski MR, van der Toorn M, Szczepanowska J. 2020; Mitochondrial network and biogenesis in response to short and long-term exposure of human BEAS-2B cells to aerosol extracts from the Tobacco Heating System 2.2. Cell Physiol Biochem. 54:230–251. DOI: 10.33594/000000216. PMID: 32153152.41. Dorn GW 2nd, Vega RB, Kelly DP. 2015; Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 29:1981–1991. DOI: 10.1101/gad.269894.115. PMID: 26443844. PMCID: PMC4604339.
Article42. Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. 2013; Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 8:e54059. DOI: 10.1371/journal.pone.0054059. PMID: 23342074. PMCID: PMC3546973.
Article43. Ramalingam L, Menikdiwela K, LeMieux M, Dufour JM, Kaur G, Kalupahana N, Moustaid-Moussa N. 2017; The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta Mol Basis Dis. 1863:1106–1114. DOI: 10.1016/j.bbadis.2016.07.019. PMID: 27497523.
Article44. Piantadosi CA, Suliman HB. 2012; Redox regulation of mitochondrial biogenesis. Free Radic Biol Med. 53:2043–2053. DOI: 10.1016/j.freeradbiomed.2012.09.014. PMID: 23000245. PMCID: PMC3604744.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nitric Oxide Increases Insulin Sensitivity in Skeletal Muscle by Improving Mitochondrial Function and Insulin Signaling
- Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells
- Exercise and Mitochondrial Remodeling in Skeletal Muscle in Type 2 Diabetes
- Effects of quercetin nanoemulsion on SIRT1 activation and mitochondrial biogenesis in the skeletal muscle of high-fat diet-fed mice
- Effects of exercise on obesity-induced mitochondrial dysfunction in skeletal muscle