Ann Lab Med.
2021 Jan;41(1):101-107. 10.3343/alm.2021.41.1.101.
Detection of Spinal Muscular Atrophy Using a Duplexed Real-Time PCR Approach With Locked Nucleic Acid-Modified Primers
- Affiliations
-
- 1Center for Medical Genetics & Hunan Key Laboratory of Medical Genetics, School of Life Sciences, Central South University, Changsha, Hunan, China
- 2Laboratory of Molecular Genetics, Hunan Jiahui Genetics Hospital, Changsha, Hunan, China
- KMID: 2512739
- DOI: http://doi.org/10.3343/alm.2021.41.1.101
Abstract
- Background
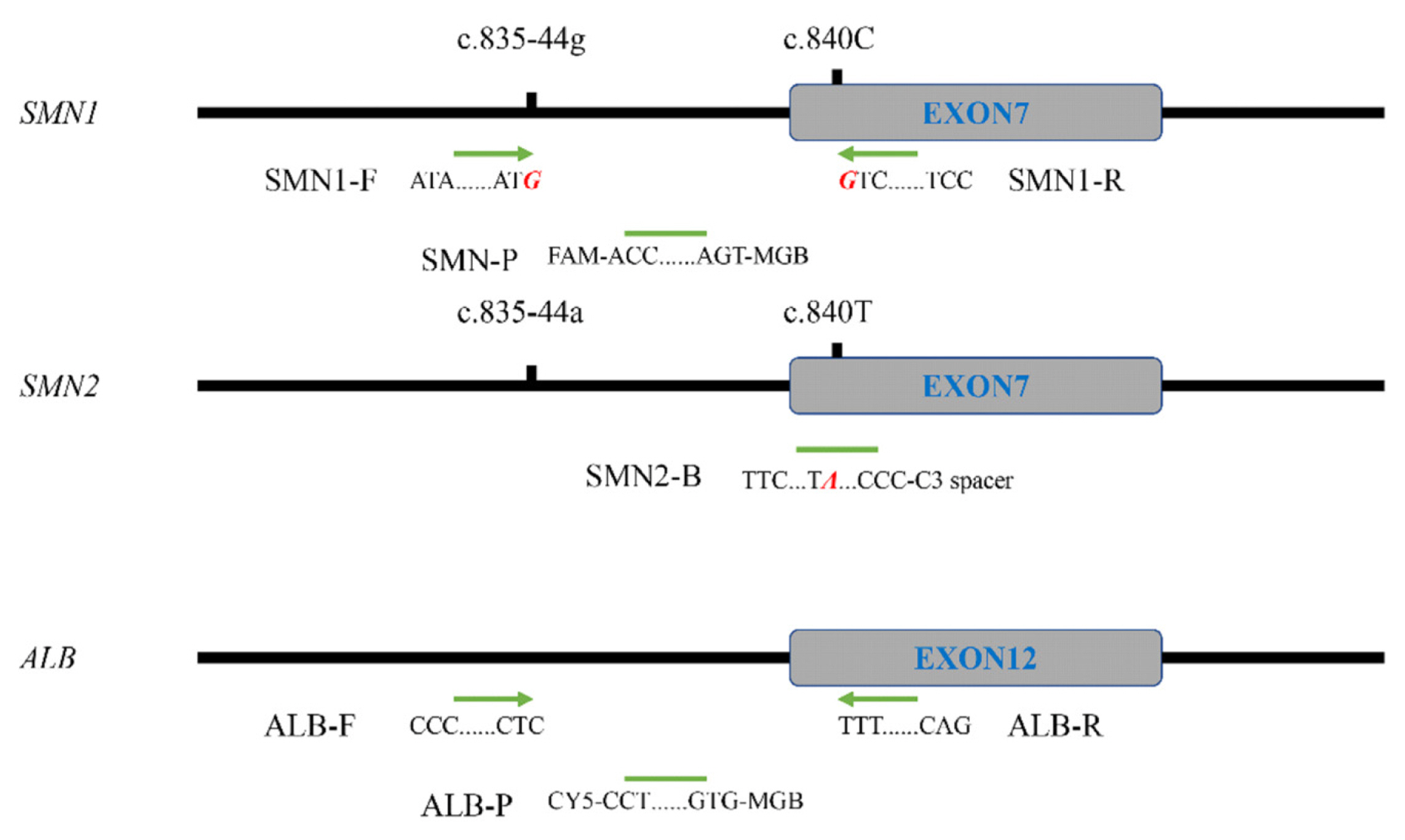
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disorder mainly caused by homozygous deletions that include exon 7 of the survival motor neuron 1 (SMN1) gene. A nearby paralog gene, SMN2, obstructs the specific detection of SMN1. We optimized a duplexed real-time PCR approach using locked nucleic acid (LNA)-modified primers to specifically detect SMN1.
Methods
An LNA-modified primer pair with 3´ ends targeting SMN1 specific sites c.835-44g and c.840C was designed, and its specificity was examined by real-time PCR and Sanger Sequencing. A duplexed real-time PCR approach for amplifying SMN1 and control gene albumin (ALB) was developed. A randomized double-blind trial with 97 fresh peripheral blood samples and 25 dried blood spots (DBS) was conducted to evaluate the clinical efficacy of the duplexed approach. This new approach was then used to screen 753 newborn DBS.
Results
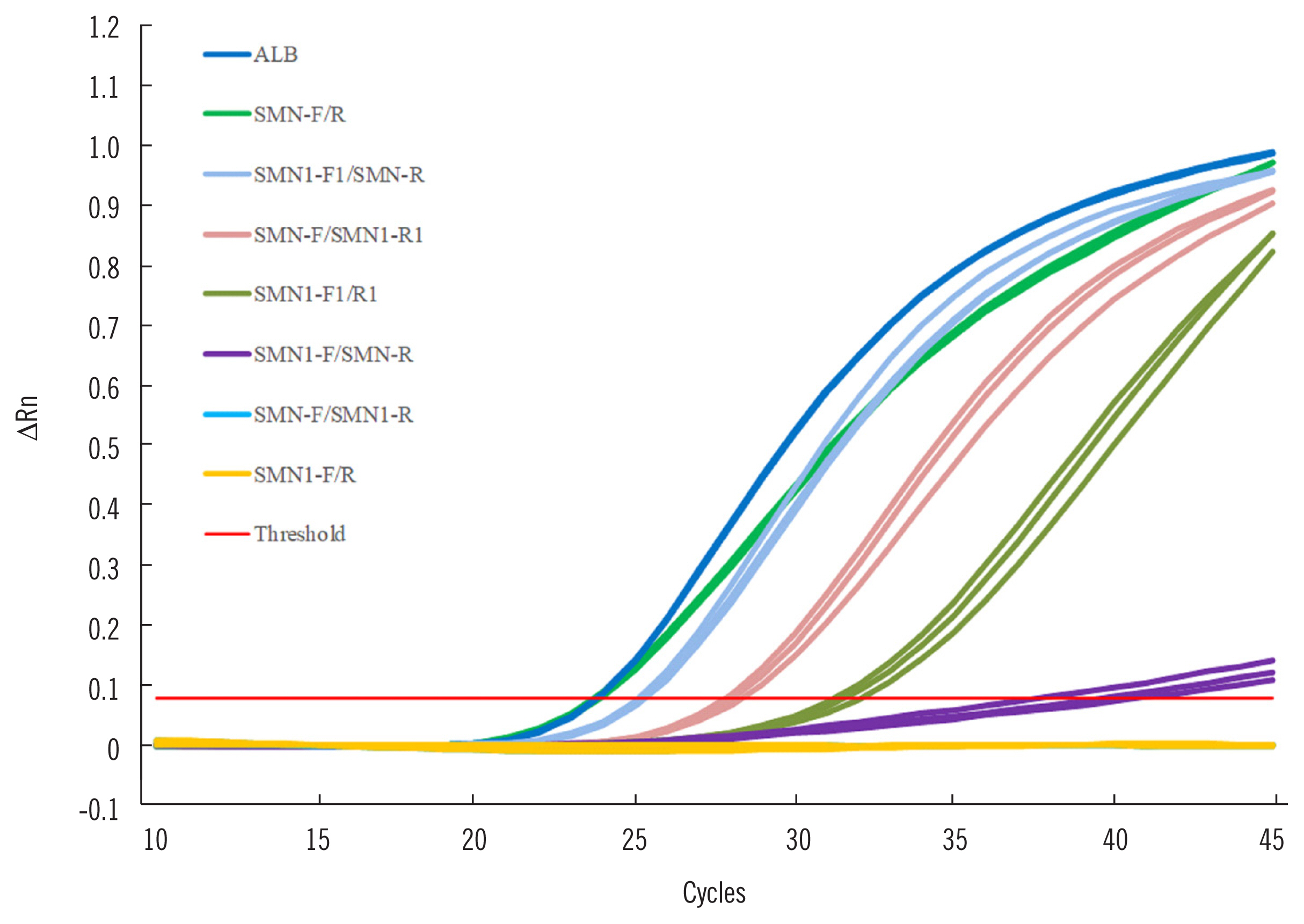
The LNA-modified primers exhibited enhanced specificity and 6.8% increased efficiency for SMN1 amplification, compared with conventional primers. After stabilizing the SMN1 test by optimizing the duplexed real-time PCR approach, a clinical trial validated that the sensitivity and specificity of our new approach for detecting SMA patients and carriers was 100%. Using this new approach, 15 of the screened 753 newborns were identified as carriers via DBS, while the rest were identified as normal individuals. These data reveal a carrier rate of 1.99% in Hunan province, South Central China.
Conclusions
We have developed a novel, specific SMN1 detection approach utilizing real-time PCR with LNA-modified primers, which could be applied to both prenatal carrier and newborn screening.
Keyword
Figure
Reference
-
1. Vill K, Kölbel H, Schwartz O, Blaschek A, Olgemöller B, Harms E, et al. One year of newborn screening for SMA–results of a German pilot project. J Neuromuscul Dis. 2019; 6:503–15.2. Hendrickson BC, Donohoe C, Akmaev VR, Sugarman EA, Labrousse P, Boguslavskiy L, et al. Differences in SMN1 allele frequencies among ethnic groups within North America. J Med Genet. 2009; 46:641–4.
Article3. Sugarman EA, Nagan N, Zhu H, Akmaev VR, Zhou Z, Rohlfs EM, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet. 2012; 20:27–32.4. Monaghan KG, Feldman GL, Palomaki GE, Spector EB. Ashkenazi Jewish Reproductive Screening Working Group, Molecular Subcommittee of the ACMG Laboratory Quality Assurance Committee. Technical standards and guidelines for reproductive screening in the Ashkenazi Jewish population. Genet Med. 2008; 10:57–72.
Article5. Grody WW, Thompson BH, Gregg AR, Bean LH, Monaghan KG, Schneider A, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013; 15:482–3.
Article6. Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995; 80:155–65.
Article7. Bürglen L, Lefebvre S, Clermont O, Burlet P, Viollet L, Cruaud C, et al. Structure and organization of the human survival motor neurone (SMN) gene. Genomics. 1996; 32:479–82.
Article8. Chan V, Yip B, Yam I, Au P, Lin CK, Wong V, et al. Carrier incidence for spinal muscular atrophy in southern Chinese. J Neurol. 2004; 251:1089–93.
Article9. Su YN, Hung CC, Lin SY, Chen FY, Chern JP, Tsai C, et al. Carrier screening for spinal muscular atrophy (SMA) in 107,611 pregnant women during the period 2005–2009: a prospective population-based cohort study. PLoS One. 2011; 6:e17067.
Article10. Wang KC, Chang CC, Chang YF, Wang SH, Chiang CK, Tsai CP. Evaluation and characterization of a high-resolution melting analysis kit for rapid carrier-screening test of spinal muscular atrophy. J Neurogenet. 2015; 29:113–6.
Article11. Lundin KE, H⊘jland T, Hansen BR, Persson R, Bramsen JB, Kjems J, et al. Biological activity and biotechnological aspects of locked nucleic acids. Adv Genet. 2013; 82:47–107.
Article12. Ishige T, Itoga S, Matsushita K. Locked nucleic acid technology for highly sensitive detection of somatic mutations in cancer. Adv Clin Chem. 2018; 83:53–72.
Article13. Edwards JG, Feldman G, Goldberg J, Gregg AR, Norton ME, Rose NC, et al. Expanded carrier screening in reproductive medicine-points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015; 125:653–62.14. American College of Obstetricians and Gynecologists. Committee opinion No. 690 summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017; 129:e35–e40.15. Sumner CJ, Crawford TO. Two breakthrough gene-targeted treatments for spinal muscular atrophy: challenges remain. J Clin Invest. 2018; 128:3219–27.
Article16. Vidal-Folch N, Gavrilov D, Raymond K, Rinaldo P, Tortorelli S, Matern D, et al. Multiplex droplet digital PCR method applicable to newborn screening, carrier status, and assessment of spinal muscular atrophy. Clin Chem. 2018; 64:1753–61.
Article17. Feng Y, Ge X, Meng L, Scull J, Li J, Tian X, et al. The next generation of population-based spinal muscular atrophy carrier screening: comprehensive pan-ethnic SMN1 copy-number and sequence variant analysis by massively parallel sequencing. Genet Med. 2017; 19:936–44.
Article18. Mishra S, Lee Y, Park JW. Direct quantification of trace amounts of a chronic myeloid leukemia biomarker using locked nucleic acid capture probes. Anal Chem. 2018; 90:12824–31.
Article19. Ishige T, Satoh M, Itoga S, Nishimura M, Matsushita K, Nomura F. High-throughput genotyping of GC (vitamin D-binding protein) by melting analysis with locked nucleic acid-incorporating dual hybridization probe for improving mismatch discrimination. Clin Chim Acta. 2018; 487:126–32.
Article20. Taylor JL, Lee FK, Yazdanpanah GK, Staropoli JF, Liu M, Carulli JP, et al. Newborn blood spot screening test using multiplexed real-time PCR to simultaneously screen for spinal muscular atrophy and severe combined immunodeficiency. Clin Chem. 2015; 61:412–9.
Article21. Chien YH, Chiang SC, Weng WC, Lee NC, Lin CJ, Hsieh WS, et al. Presymptomatic diagnosis of spinal muscular atrophy through newborn screening. J Pediatr. 2017; 190:124–9.e1.
Article22. Gong B, Zhang L, Hou YP, Hu HY, Li HC, Tan MY, et al. Carrier screening for spinal muscular atrophy in 4719 pregnant women in Shanghai region [Article in Chinese]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013; 30:670–2.23. Park JE, Yun SA, Roh EY, Yoon JH, Shin S, Ki CS. Carrier frequency of spinal muscular atrophy in a large-scale Korean population. Ann Lab Med. 2020; 40:326–30.
Article24. Mailman MD, Hemingway T, Darsey RL, Glasure CE, Huang Y, Chadwick RB, et al. Hybrids monosomal for human chromosome 5 reveal the presence of a spinal muscular atrophy (SMA) carrier with two SMN1 copies on one chromosome. Hum Genet. 2001; 108:109–15.
Article25. Wei X, Tan H, Yang P, Zhang R, Tan B, Zhang Y, et al. Notable carrier risks for individuals having two copies of SMN1 in spinal muscular atrophy families with 2-copy alleles: estimation based on Chinese meta-analysis data. J Genet Couns. 2017; 26:72–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Camptocormia Due to Selective Paraspinal Muscle Atrophy
- Evaluation of Artus HBV LC PCR kit using SLAN Real-time PCR
- Diagnosis of Viral Infection Using Real-time Polymerase Chain Reaction
- Automated Nucleic Acid Extraction Systems for Detecting Cytomegalovirus and Epstein-Barr Virus Using Real-Time PCR: A Comparison Study Between the QIAsymphony RGQ and QIAcube Systems
- A Case of Spinal Muscular Atrophy with Hypertrophy of Calf-Muscles