Ann Lab Med.
2021 May;41(3):259-267. 10.3343/alm.2021.41.3.259.
Immune Checkpoint Programmed Cell Death Protein-1 (PD-1) Expression on Bone Marrow T Cell Subsets in Patients With Plasma Cell Myeloma
- Affiliations
-
- 1Department of Laboratory Medicine, Kyung Hee University School of Medicine and Kyung Hee University Hospital, Gangdong, Seoul, Korea
- 2Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Inje University College of Medicine, Busan Baik Hospital, Busan, Korea
- 4Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2512683
- DOI: http://doi.org/10.3343/alm.2021.41.3.259
Abstract
- Background
Plasma cell myeloma (PCM) is caused by immune dysregulation. We evaluated the expression of immune checkpoint programmed cell death protein-1 (PD-1) on T cell subsets in PCM patients according to disease course and cytogenetic abnormalities. This study aimed to find a target group suitable for therapeutic use of PD-1 blockade in PCM.
Methods
A total of 188 bone marrow (BM) samples from 166 PCM patients and 32 controls were prospectively collected between May 2016 and May 2017. PD-1 expression on BM T cell subsets was measured using flow cytometry.
Results
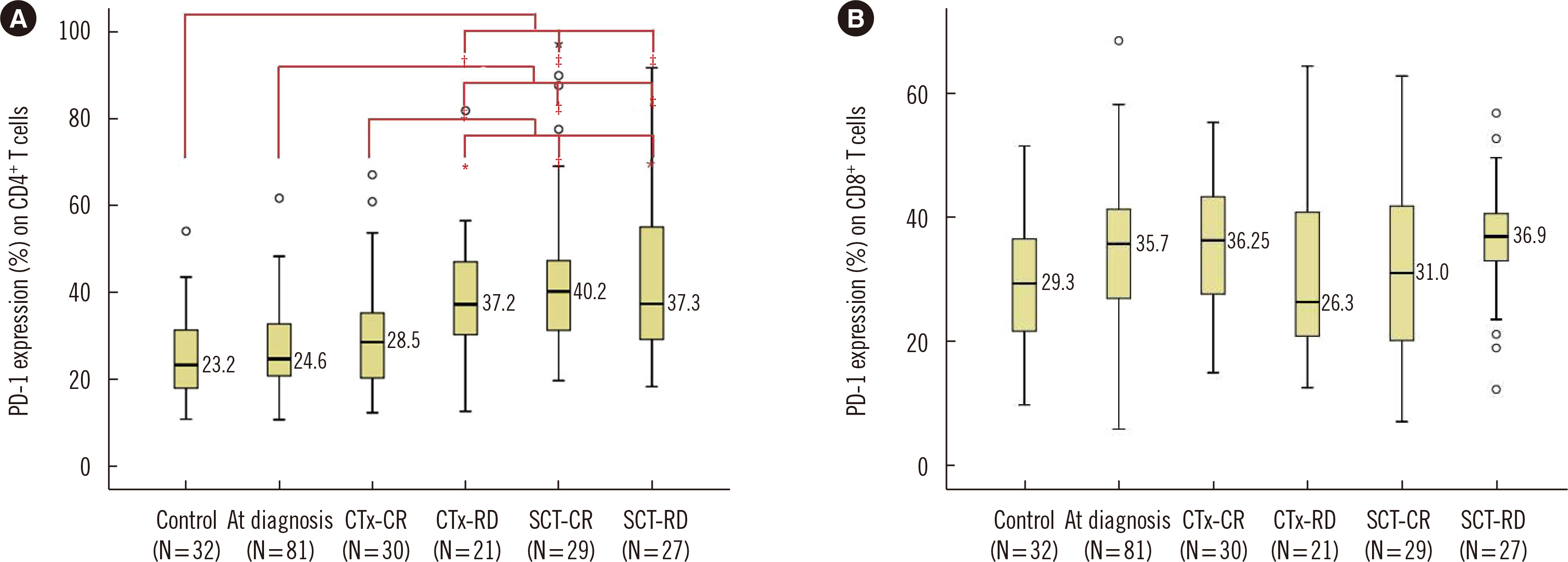
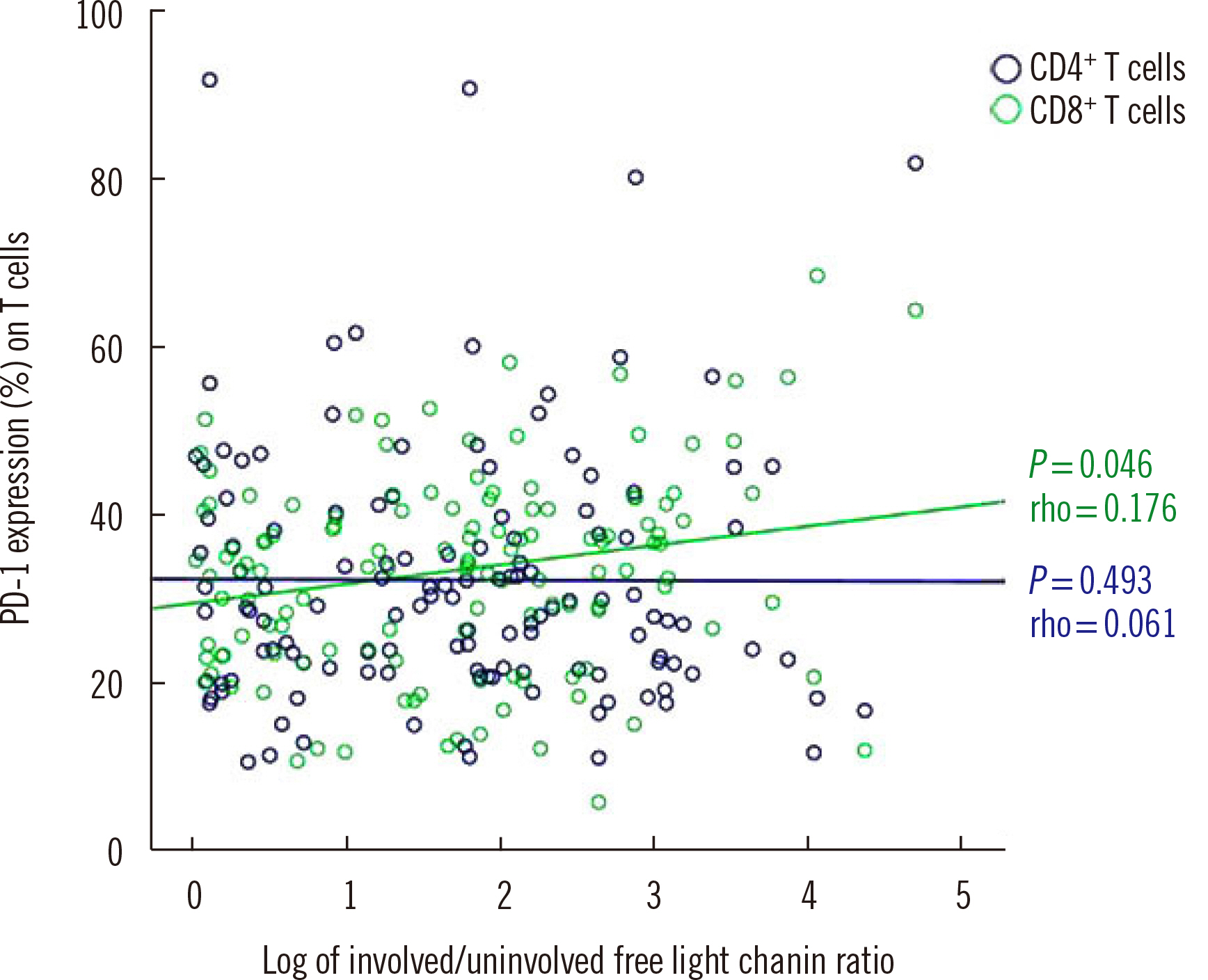
At diagnosis, the median PD-1 expression on CD4+ T cells was 24.6%, which did not significantly differ from that in controls. After stem cell transplantation, PD-1 expression on CD4+ T cells was higher than that at diagnosis (P < 0.001), regardless of residual disease. PD-1 expression on CD4+ T cells in patients with residual disease after chemotherapy was significantly higher than that at diagnosis (P = 0.001) and after complete remission following chemotherapy (P = 0.044). PD-1 expression on CD8+ T cells was higher in PCM patients with cytogenetic abnormalities, including monosomy 13, 1q gain, complex karyotype, and hypodiploidy.
Conclusions
PD-1 blockade might have therapeutic potential in refractory PCM patients after chemotherapy, especially in those with high- or intermediate-risk cytogenetic abnormalities.
Keyword
Figure
Reference
-
1. Patel SP, Kurzrock R. 2015; PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 14:847–56. DOI: 10.1158/1535-7163.MCT-14-0983. PMID: 25695955.
Article2. Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. 2016; Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 34:2698–704. DOI: 10.1200/JCO.2015.65.9789. PMID: 27269947. PMCID: PMC5019749.
Article3. Usmani SZ, Schjesvold F, Rocafiguera AO, Karlin L, Rifkin RM, Yimer HA, et al. 2018; A phase 3 randomized study of pembrolizumab (pembro) plus lenalidomide (len) and low-dose dexamethasone (Rd) versus Rd for newly diagnosed and treatment-naive multiple myeloma (MM): Keynote-185. J Clin Oncol. 36:8010. DOI: 10.1200/JCO.2018.36.15_suppl.8010.
Article4. Mateos MV, Blacklock H, Schjesvold F, Rocafiguera AO, Simpson D, George A, et al. 2018; A phase 3 randomized study of pembrolizumab (Pembro) plus pomalidomide (Pom) and dexamethasone (Dex) for relapsed/refractory multiple myeloma (RRMM): Keynote-183. J Clin Oncol. 36:8021. DOI: 10.1200/JCO.2018.36.15_suppl.8021.
Article5. Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosino L, et al. 2015; Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 33:2863–9. DOI: 10.1200/JCO.2015.61.2267. PMID: 26240224. PMCID: PMC4846284.
Article6. Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR, et al. 2013; Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 88:360–76. DOI: 10.1016/j.mayocp.2013.01.019. PMID: 23541011.
Article7. Health Insurance Review & Assessment Service. Healthcare big data hub. http://opendata.hira.or.kr/op/opc/olap3thDsInfo.do. Updated on Jan 2020.8. Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. 2008; Improved survival in multiple myeloma and the impact of novel therapies. Blood. 111:2516–20. DOI: 10.1182/blood-2007-10-116129. PMID: 17975015. PMCID: PMC2254544.
Article9. Lee HY, Park CJ, Ahn A, Lee MY, Cho YU, Jang S, Seo EJ, Lee KH, Lee JH, Lee HY, et al. 2019; Two Rare Cases of Therapy-Related Acute Lymphoblastic Leukemia in Patients With Plasma Cell Myeloma. Ann Lab Med. 39:496–8. DOI: 10.3343/alm.2019.39.5.496. PMID: 31037870. PMCID: PMC6502952.
Article10. Paiva B, Azpilikueta A, Puig N, Ocio EM, Sharma R, Oyajobi BO, et al. 2015; PD-L1/PD-1 presence in the tumor microenvironment and activity of PD-1 blockade in multiple myeloma. Leukemia. 29:2110–3. DOI: 10.1038/leu.2015.79. PMID: 25778100.
Article11. Stathopoulou C, Gangaplara A, Mallett G, Flomerfelt FA, Liniany LP, Knight D, et al. 2018; PD-1 inhibitory receptor downregulates asparaginyl endopeptidase and maintains Foxp3 transcription factor stability in induced regulatory T cells. Immunity. 49:247–63. e7. DOI: 10.1016/j.immuni.2018.05.006. PMID: 30054205. PMCID: PMC6105434.
Article12. Amarnath S, Costanzo CM, Mariotti J, Ullman JL, Telford WG, Kapoor V, et al. 2010; Regulatory T cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS Biol. 8:e1000302. DOI: 10.1371/journal.pbio.1000302. PMID: 20126379. PMCID: PMC2814822.
Article13. Amarnath S, Chen H, Foley JE, Costanzo CM, Sennesh JD, Solomon MA, et al. 2011; Host-based Th2 cell therapy for prolongation of cardiac allograft viability. PLoS One. 6:e18885. DOI: 10.1371/journal.pone.0018885. PMID: 21559526. PMCID: PMC3084712.
Article14. Muthu Raja KR, Rihova L, Zahradova L, Klincova M, Penka M, Hajek R. 2012; Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS One. 7:e47077. DOI: 10.1371/journal.pone.0047077. PMID: 23071717. PMCID: PMC3468567.
Article15. Karnell FG, Lin D, Motley S, Duhen T, Lim N, Campbell DJ, et al. 2017; Reconstitution of immune cell populations in multiple sclerosis patients after autologous stem cell transplantation. Clin Exp Immunol. 189:268–78. DOI: 10.1111/cei.12985. PMID: 28498568. PMCID: PMC5543487.
Article16. Arruda LCM, de Azevedo JTC, de Oliveira GLV, Scortegagna GT, Rodrigues ES, Palma PVB, et al. 2016; Immunological correlates of favorable long-term clinical outcome in multiple sclerosis patients after autologous hematopoietic stem cell transplantation. Clin Immunol. 169:47–57. DOI: 10.1016/j.clim.2016.06.005. PMID: 27318116.
Article17. Simonetta F, Pradier A, Bosshard C, Masouridi-Levrat S, Dantin C, Koutsi A, et al. 2019; Dynamics of expression of programmed cell death protein-1 (PD-1) on T cells after allogeneic hematopoietic stem cell transplantation. Front Immunol. 10:1034. DOI: 10.3389/fimmu.2019.01034. PMID: 31156625. PMCID: PMC6531929.
Article18. Merindol N, Champagne MA, Duval M, Soudeyns H. 2011; CD8(+) T-cell reconstitution in recipients of umbilical cord blood transplantation and characteristics associated with leukemic relapse. Blood. 118:4480–8. DOI: 10.1182/blood-2011-04-349241. PMID: 21813446.
Article19. Pawlyn C, Morgan GJ. 2017; Evolutionary biology of high-risk multiple myeloma. Nat Rev Cancer. 17:543–56. DOI: 10.1038/nrc.2017.63. PMID: 28835722.
Article20. Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, Alessandro R. 2013; Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci. 14:5338–66. DOI: 10.3390/ijms14035338. PMID: 23466882. PMCID: PMC3634447.
Article21. Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, et al. 2013; BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 123:1542–55. DOI: 10.1172/JCI66517. PMID: 23454749. PMCID: PMC3613927.
Article22. Raimondi L, De Luca A, Amodio N, Manno M, Raccosta S, Taverna S, et al. 2015; Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget. 6:13772–89. DOI: 10.18632/oncotarget.3830. PMID: 25944696. PMCID: PMC4537049.
Article23. Lemaire M, Deleu S, De Bruyne E, Van Valckenborgh E, Menu E, Vanderkerken K. 2011; The microenvironment and molecular biology of the multiple myeloma tumor. Adv Cancer Res. 110:19–42. DOI: 10.1016/B978-0-12-386469-7.00002-5. PMID: 21704227.
Article24. Neri P, Ren L, Azab AK, Brentnall M, Gratton K, Klimowicz AC, et al. 2011; Integrin β7-mediated regulation of multiple myeloma cell adhesion, migration, and invasion. Blood. 117:6202–13. DOI: 10.1182/blood-2010-06-292243. PMID: 21474670. PMCID: PMC3122944.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiotherapy and immune checkpoint blockades: a snapshot in 2016
- Cutaneous Adverse Events of Anti-Programmed Cell Death Receptor-1 Antibody: Two Case Reports and a Literature Review
- Immune-Checkpoint Inhibitors in the Era of Precision Medicine: What Radiologists Should Know
- SOLITARY PLASMA CELL MYELOMA ON ANTERIOR MAXILLA: A CASE REPORT
- Analysis of the Expression and Regulation of PD-1 Protein on the Surface of Myeloid-Derived Suppressor Cells (MDSCs)