Ann Lab Med.
2021 Mar;41(2):171-180. 10.3343/alm.2021.41.2.171.
Laboratory Diagnostic Methods for Clostridioides difficile Infection: the First Systematic Review and Meta-analysis in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Laboratory Medicine, Sanggye Paik Hospital, School of Medicine, Inje University, Seoul, Korea
- KMID: 2512681
- DOI: http://doi.org/10.3343/alm.2021.41.2.171
Abstract
- Background
Various methods are used for the diagnosis of Clostridioides difficile infection (CDI). We systematically analyzed and investigated the performance of current laboratory diagnostic methods for CDI.
Methods
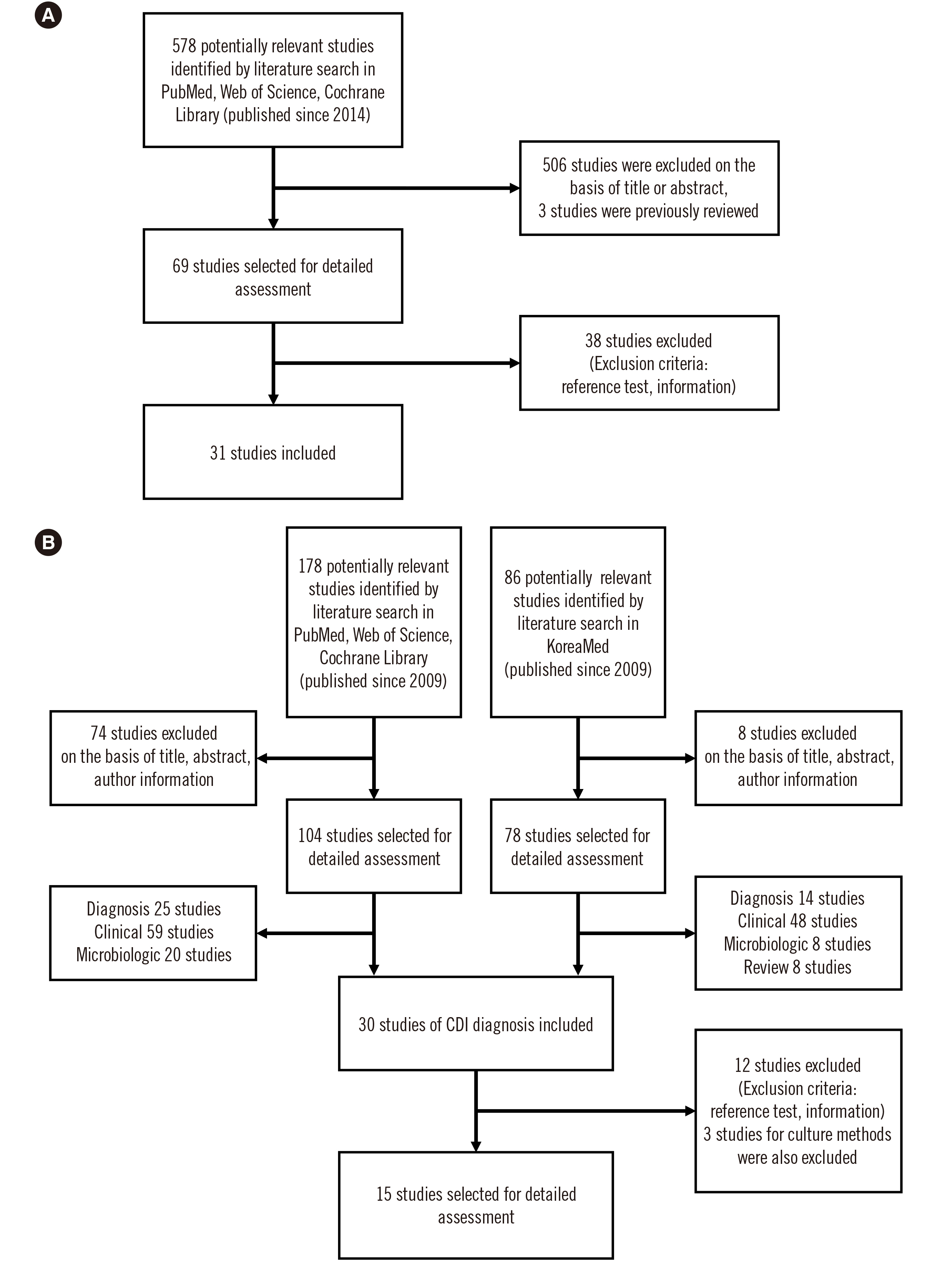
We performed systematic review and meta-analysis of studies in PubMed, Web of Science, Cochrane Library, and KoreaMed. The following methods were evaluated: glutamate dehydrogenase (GDH) enzyme immunoassays (GDH EIAs), toxin A and B detection by enzyme immunoassays (toxin AB EIAs), and nucleic acid amplification tests (NAATs) for C. difficile toxin genes. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each method were calculated.
Results
Based on 39 studies, the pooled sensitivities/specificities were 92.7%/94.6%, 57.9%/97.0%, and 90.0%/95.8% for GDH EIAs, toxin AB EIAs, and NAATs, respectively, compared with those of toxigenic culture. The pooled sensitivities of automated EIAs were significantly higher than those of non-automated EIAs for both GDH and toxins A and B. The pooled sensitivity of Xpert C. difficile was significantly higher than those of other NAATs. PPVs increased as CDI prevalence increased, and NPVs were excellent when CDI prevalence was low; at CDI prevalence of 5%, PPV = 37%–65% and NPV = 97%–100%; at CDI prevalence of 50%, PPV = 92%–97% and NPV = 65%–98%.
Conclusions
Toxin AB EIAs still show unsatisfactory sensitivity, whereas GDH EIAs and NAATs show relatively high sensitivity. However, toxin AB EIAs are the most specific tests. This study may provide useful information for CDI diagnosis.
Keyword
Figure
Reference
-
1. Leffler DA, Lamont JT. 2015; Clostridium difficile infection. N Engl J Med. 372:1539–48. DOI: 10.1056/NEJMra1403772. PMID: 25875259.2. Bagdasarian N, Rao K, Malani PN. 2015; Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 313:398–408. DOI: 10.1001/jama.2014.17103. PMID: 25626036. PMCID: PMC6561347.3. Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, et al. 2016; European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect. 22(S4):S63–81. DOI: 10.1016/j.cmi.2016.03.010. PMID: 27460910.4. McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. 2018; Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 66:987–94. DOI: 10.1093/cid/ciy149. PMID: 29562266.5. Chung HS, Park JS, Shin BM. 2019; Laboratory diagnosis of Clostridium difficile infection in Korea: the first national survey. Ann Lab Med. 39:317–21. DOI: 10.3343/alm.2019.39.3.317. PMID: 30623624.6. Alcalá L, Reigadas E, Marin M, Fernández-Chico A, Catalan P, Bouza E. 2015; Comparison of GenomEra C. difficile and Xpert C. difficile as confirmatory tests in a multistep algorithm for diagnosis of Clostridium difficile infection. J Clin Microbiol. 53:332–5. DOI: 10.1128/JCM.03093-14. PMID: 25392360.7. Banz A, Lantz A, Riou B, Foussadier A, Miller M, Davies K, et al. 2018; Sensitivity of single-molecule array assays for detection of Clostridium difficile toxins in comparison to conventional laboratory testing algorithms. J Clin Microbiol. 56:e00452–18. DOI: 10.1128/JCM.00452-18. PMID: 29898996. PMCID: PMC6062787.
Article8. Beck ET, Buchan BW, Riebe KM, Alkins BR, Pancholi P, Granato PA, et al. 2014; Multicenter evaluation of the Quidel Lyra Direct C. difficile nucleic acid amplification assay. J Clin Microbiol. 52:1998–2002. DOI: 10.1128/JCM.03089-13. PMID: 24671790. PMCID: PMC4042815.9. Blaich A, Frei R, Castellano C, Kiessling C, Geschke A, Rentsch KM, et al. 2017; Evaluation of two novel chemiluminescence immunoassays for the detection of Clostridium difficile glutamate dehydrogenase and toxin A&B. J Microbiol Methods. 135:63–5. DOI: 10.1016/j.mimet.2017.02.004. PMID: 28192156.10. Carson KC, Riley TV. 2016; Comparison of the Vidas C. difficile and Quik Chek-60 glutamate dehydrogenase assays for the detection of Clostridium difficile in faecal samples. Pathology. 48:506–8. DOI: 10.1016/j.pathol.2016.04.007. PMID: 27311869.11. Cheng JW, Xiao M, Kudinha T, Xu ZP, Sun LY, Hou X, et al. 2015; The role of glutamate dehydrogenase (GDH) testing assay in the diagnosis of Clostridium difficile infections: a high sensitive screening test and an essential step in the proposed laboratory diagnosis workflow for developing countries like China. PLoS One. 10:e0144604. DOI: 10.1371/journal.pone.0144604. PMID: 26659011.12. Davies KA, Berry CE, Morris KA, Smith R, Young S, Davis TE, et al. 2015; Comparison of the Vidas C. difficile GDH automated enzyme-linked fluorescence immunoassay (ELFA) with another commercial enzyme immunoassay (EIA) (Quik Chek-60), two selective media, and a PCR assay for gluD for detection of Clostridium difficile in fecal samples. J Clin Microbiol. 53:1931–4. DOI: 10.1128/JCM.00649-15. PMID: 25788549.13. Gomez EJ, Montgomery S, Alby K, Robinson DP, Roundtree SS, Blecker-Shelly D, et al. 2018; Poor yield of Clostridium difficile testing algorithms using glutamate dehydrogenase antigen and C. difficile toxin enzyme immunoassays in a pediatric population with declining prevalence of clostridium difficile strain BI/NAP1/027. Diagn Microbiol Infect Dis. 91:229–32. DOI: 10.1016/j.diagmicrobio.2018.02.016. PMID: 29567127.14. Hong G, Park KS, Ki CS, Lee NY. 2014; Evaluation of the illumigene C. difficile assay for toxigenic Clostridium difficile detection: a prospective study of 302 consecutive clinical fecal samples. Diagn Microbiol Infect Dis. 80:177–80. DOI: 10.1016/j.diagmicrobio.2014.08.014. PMID: 25241189.15. Jazmati N, Wiegel P, Ličanin B, Plum G. 2015; Evaluation of the Qiagen artus C. difficile QS-RGQ kit for detection of Clostridium difficile toxins A and B in clinical stool specimens. J Clin Microbiol. 53:1942–4. DOI: 10.1128/JCM.00613-15. PMID: 25809977.16. Jensen MB, Olsen KE, Nielsen XC, Hoegh AM, Dessau RB, Atlung T, et al. 2015; Diagnosis of Clostridium difficile: real-time PCR detection of toxin genes in faecal samples is more sensitive compared to toxigenic culture. Eur J Clin Microbiol Infect Dis. 34:727–36. DOI: 10.1007/s10096-014-2284-7. PMID: 25421216.17. Johansson K, Karlsson H, Norén T. 2016; Clostridium difficile infection diagnostics - evaluation of the C. DIFF Quik Chek Complete assay, a rapid enzyme immunoassay for detection of toxigenic C. difficile in clinical stool samples. APMIS. 124:1016–20. DOI: 10.1111/apm.12595. PMID: 27651167.18. Kilic A, Alam MJ, Tisdel NL, Shah DN, Yapar M, Lasco TM, et al. 2015; Multiplex real-time PCR method for simultaneous identification and toxigenic type characterization of Clostridium difficile from stool samples. Ann Lab Med. 35:306–13. DOI: 10.3343/alm.2015.35.3.306. PMID: 25932438. PMCID: PMC4390698.19. Kim H, Kim WH, Kim M, Jeong SH, Lee K. 2014; Evaluation of a rapid membrane enzyme immunoassay for the simultaneous detection of glutamate dehydrogenase and toxin for the diagnosis of Clostridium difficile infection. Ann Lab Med. 34:235–9. DOI: 10.3343/alm.2014.34.3.235. PMID: 24790912.20. Kim H, Jeong SH, Kim M, Lee Y, Lee K. 2012; Detection of Clostridium difficile toxin A/B genes by multiplex real-time PCR for the diagnosis of C. difficile infection. J Med Microbiol. 61:274–7. DOI: 10.1099/jmm.0.035618-0. PMID: 21959205.21. Kosai K, Iwanaga Y, Akamatsu N, Okada Y, Kaku N, Uno N, et al. 2017; Performance evaluation of the Verigene® Clostridium difficile nucleic acid test, an automated multiplex molecular testing system for detection of C. difficile toxin. J Infect Chemother. 23:674–7. DOI: 10.1016/j.jiac.2017.07.002. PMID: 28751156.22. Legaria MC, Rollet R, Di Martino A, Castello L, Barberis C, Rossetti MA, et al. 2018; Detection of toxigenic Clostridioides [Clostridium] difficile: Usefulness of two commercially available enzyme immunoassays and a PCR assay on stool samples and stool isolates. Rev Argent Microbiol. 50:36–44. DOI: 10.1016/j.ram.2017.01.002. PMID: 28988901.23. Makristathis A, Zeller I, Mitteregger D, Kundi M, Hirschl AM. 2017; Comprehensive evaluation of chemiluminescent immunoassays for the laboratory diagnosis of Clostridium difficile infection. Eur J Clin Microbiol Infect Dis. 36:1253–9. DOI: 10.1007/s10096-017-2916-9. PMID: 28181032. PMCID: PMC5495843.24. Moon HW, Kim HN, Kim JY, Hur M, Kim H, Yun YM. 2017; Performance of the artus C. difficile QS-RGQ Kit for the detection of toxigenic Clostridium difficile. Clin Biochem. 50:84–7. DOI: 10.1016/j.clinbiochem.2016.08.013. PMID: 27556286.25. Moon HW, Kim HN, Hur M, Shim HS, Kim H, Yun YM. 2016; Comparison of diagnostic algorithms for detecting toxigenic Clostridium difficile in routine practice at a tertiary referral hospital in Korea. PLoS One. 11:e0161139. DOI: 10.1371/journal.pone.0161139. PMID: 27532104.26. Morinaga Y, Akamatsu N, Matsuda J, Tateno H, Tomaru T, Tanaka A, et al. 2018; Diagnostic utilities of a fully automated molecular test for toxigenic Clostridium difficile. J Infect Chemother. 24:88–91. DOI: 10.1016/j.jiac.2017.09.003. PMID: 28974364.27. Neuendorf M, Guadarrama-Gonzalez R, Lamik B, MacKenzie CR. 2016; A prospective study of two isothermal amplification assays compared with real-time PCR, CCNA and toxigenic culture for the diagnosis of Clostridium difficile infection. BMC Microbiol. 16:19. DOI: 10.1186/s12866-016-0635-5. PMID: 26868647. PMCID: PMC4751656.
Article28. Olling A, Leidinger H, Hoffmann R. 2016; Comparison of enzyme immunoassays and rapid diagnostic tests for Clostridium difficile glutamate dehydrogenase and toxin a+B to toxinogenic culture on a highly selective chromogenic medium. Eur J Clin Microbiol Infect Dis. 35:1655–9. DOI: 10.1007/s10096-016-2706-9. PMID: 27323765.29. Paitan Y, Miller-Roll T, Adler A. 2017; Comparative performance study of six commercial molecular assays for rapid detection of toxigenic Clostridium difficile. Clin Microbiol Infect. 23:567–72. DOI: 10.1016/j.cmi.2017.02.016. PMID: 28223147.30. Peterson LR, Young SA, Davis TE. Jr. 2017; , Wang ZX, Duncan J, Noutsios C, et al. Evaluation of the cobas Cdiff test for detection of toxigenic Clostridium difficile in stool samples. J Clin Microbiol. 55:3426–36. DOI: 10.1128/JCM.01135-17. PMID: 28954901.31. Putsathit P, Morgan J, Bradford D, Engelhardt N, Riley TV. 2015; Evaluation of the BD Max Cdiff assay for the detection of toxigenic Clostridium difficile in human stool specimens. Pathology. 47:165–8. DOI: 10.1097/PAT.0000000000000214. PMID: 25551308.32. Rajabally N, Kullin B, Ebrahim K, Brock T, Weintraub A, Whitelaw A, et al. 2016; A comparison of Clostridium difficile diagnostic methods for identification of local strains in a South African centre. J Med Microbiol. 65:320–7. DOI: 10.1099/jmm.0.000231. PMID: 26860329.33. Seo JY, Jeong JH, Kim KH, Ahn JY, Park PW, Seo YH. 2017; Laboratory diagnosis of Clostridium difficile infection: Comparison of Techlab C. diff Quik Chek Complete, Xpert C. difficile, and multistep algorithmic approach. J Clin Lab Anal. 31:e22135. DOI: 10.1002/jcla.22135. PMID: 28177534. PMCID: PMC6817171.34. Shin BM, Lee EJ, Moon JW, Lee SY. 2016; Evaluation of the VIDAS glutamate dehydrogenase assay for the detection of Clostridium difficile. Anaerobe. 40:68–72. DOI: 10.1016/j.anaerobe.2016.06.001. PMID: 27282799.35. Shin BM, Yoo SM, Shin WC. 2016; Evaluation of Xpert C. difficile, BD MAX Cdiff, IMDx C. difficile for Abbott m2000, and Illumigene C. difficile assays for direct detection of toxigenic Clostridium difficile in stool specimens. Ann Lab Med. 36:131–7. DOI: 10.3343/alm.2016.36.2.131. PMID: 26709260. PMCID: PMC4713846.36. Shin BM, Mun SJ, Yoo SJ, Kuak EY. 2012; Comparison of BD GeneOhm Cdiff and Seegene Seeplex ACE PCR assays using toxigenic Clostridium difficile culture for direct detection of tcdB from stool specimens. J Clin Microbiol. 50:3765–7. DOI: 10.1128/JCM.01440-12. PMID: 22952270. PMCID: PMC3486254.37. Shin S, Kim M, Kim M, Lim H, Kim H, Lee K, et al. 2012; Evaluation of the Xpert Clostridium difficile assay for the diagnosis of Clostridium difficile infection. Ann Lab Med. 32:355–8. DOI: 10.3343/alm.2012.32.5.355. PMID: 22950071. PMCID: PMC3427823.38. Shin BM, Lee EJ, Kuak EY, Yoo SJ. 2009; Comparison of VIDAS CDAB and CDA immunoassay for the detection of Clostridium difficile in a tcdA- tcdB+ C. difficile prevalent area. Anaerobe. 15:266–9. DOI: 10.1016/j.anaerobe.2009.09.008. PMID: 19772927.39. Shin BM, Kuak EY, Lee EJ, Songer JG. 2009; Algorithm combining toxin immunoassay and stool culture for diagnosis of Clostridium difficile infection. J Clin Microbiol. 47:2952–6. DOI: 10.1128/JCM.00609-09. PMID: 19625481. PMCID: PMC2738110.40. Shin BM, Yoo SJ, Oh HJ. 2009; Comparison of two enzyme immunoassay for detection of Clostridium difficile toxin A and toxin B. Korean J Lab Med. 29:122–6. DOI: 10.3343/kjlm.2009.29.2.122. PMID: 19411778.41. Silva RO, Vilela EG, Neves MS, Lobato FC. 2014; Evaluation of three enzyme immunoassays and a nucleic acid amplification test for the diagnosis of Clostridium difficile-associated diarrhea at a university hospital in Brazil. Rev Soc Bras Med Trop. 47:447–50. DOI: 10.1590/0037-8682-0100-2014. PMID: 25229284.42. Soh YS, Yang JJ, You E, La Jeon Y, Kim MJ, Nam YS, et al. 2014; Comparison of two molecular methods for detecting toxigenic Clostridium difficile. Ann Clin Lab Sci. 44:27–31. PMID: 24695470.43. Tojo M, Nagamatsu M, Hayakawa K, Mezaki K, Kirikae T, Ohmagari N. 2014; Evaluation of an automated rapid diagnostic test for detection of Clostridium difficile. PLoS One. 9:e106102. DOI: 10.1371/journal.pone.0106102. PMID: 25170836. PMCID: PMC4149505.44. Yoo J, Lee H, Park KG, Lee GD, Park YG, Park YJ. 2015; Evaluation of 3 automated real-time PCR (Xpert C. difficile assay, BD MAX Cdiff, and IMDx C. difficile for Abbott m2000 assay) for detecting Clostridium difficile toxin gene compared to toxigenic culture in stool specimens. Diagn Microbiol Infect Dis. 83:7–10. DOI: 10.1016/j.diagmicrobio.2015.05.005. PMID: 26081240.45. Jhang JS, Lifshitz MS. Mcpherson RA, Pincus MR, editors. 2016. Postanalysis: Medical Decision Making. Henry's clinical diagnosis and management by laboratory methods. 23rd ed. Elsevier, Saunders;Philadelphia: p. 73–83.46. Crobach MJ, Dekkers OM, Wilcox MH, Kuijper EJ. 2009; European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin Microbiol Infect. 15:1053–66. DOI: 10.1111/j.1469-0691.2009.03098.x. PMID: 19929972.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laboratory diagnosis of Clostridioides difficile infection: guidelines and status of practice in Korea
- Which is the Preferred Regimen for Non-Severe Clostridioides difficile Infection in Korea, Vancomycin or Metronidazole?
- Diagnostic Stewardship on the Extra-Analytical Phase to Secure Quality Assurance of Diagnostic Tests for Clostridioides difficile Infection
- The Trend of Clostridioides difficile Infection in Korean Hospitals with the Analysis of Nationwide Sample Cohort
- Clostridioides Infection in Patients with Inflammatory Bowel Disease