Lab Med Online.
2020 Oct;10(4):314-320. 10.47429/lmo.2020.10.4.314.
Utility of Plasma Cell Screening Tube Kit to Differentiate Neoplastic Plasma Cells from Reactive Plasma Cells
- Affiliations
-
- 1Department of Laboratory Medicine, Soonchunhyang University Seoul Hospital, Seoul, Korea
- KMID: 2512271
- DOI: http://doi.org/10.47429/lmo.2020.10.4.314
Abstract
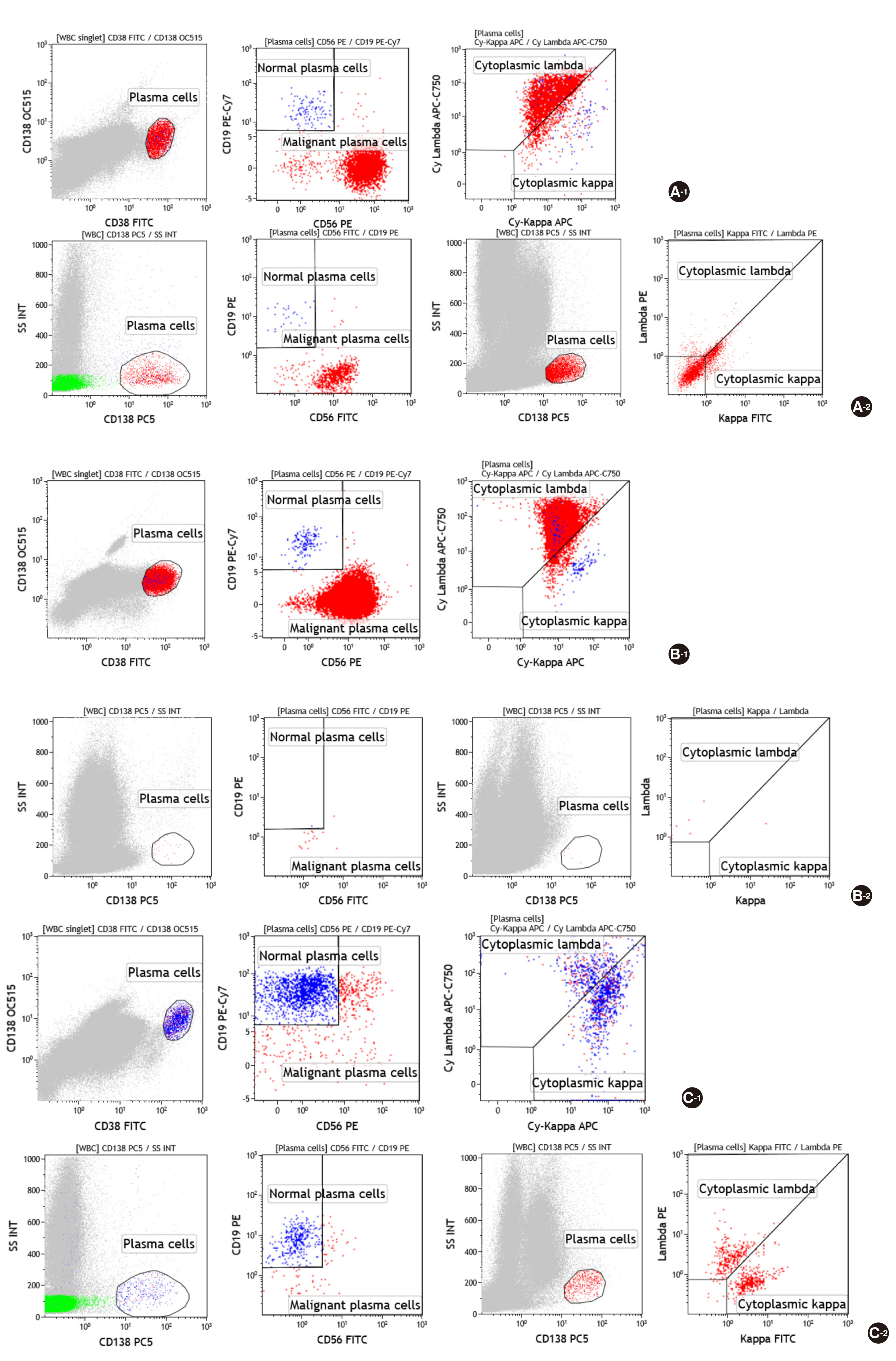
- Flow cytometry is a powerful tool for analysis of hematologic malignancies, that provides rapid, quantitative, and multiparametric analysis of heterogeneous cell populations, but requires standardization because of complexities in panel design and interpretation. Here, we compared the Plasma Cell Screening Tube (PCST) kit (Cytognos, Spain) in conjunction with EuroFlow antibody panels for standardization of flow cytometry to a conventional method for diagnosis of plasma cell dyscrasias. Thirty-nine bone marrow samples and one peripheral blood sample from 40 patients were tested. Thirty-three patients were diagnosed with multiple myeloma (MM), and seven were in a reactive state. In PCST implementation, eight antibodies were used for staining, including anti-CD45-Pacific Blue, anti-CD19-PECy7, anti-CD138-OC515, anti-CD38-FITC, anti-CD56-PE, anti-β2-microglobulin-PerCPCy5.5, anti-kappa-APC, and anti-lambda-APC-C750. Plasma cells were initially identified using CD38 and CD138; thereafter, CD38+, CD138+ gated cells were analyzed for CD56, CD19, CD45, cytoplasmic kappa, cytoplasmic lambda, and β2-microglobulin. Conventional flow cytometry was performed with six monoclonal antibodies, including anti-CD56-FITC, anti-kappa-FITC, anti-CD19-PE, anti-lambda-PE, anti-CD138-PECy5, and anti-CD45-PECy7 (Beckman Coulter, USA). Monoclonal plasma cells with cytoplasmic light-chain restriction were detected in 30 of 33 (90.9%) MM cases by conventional methods, and 32 of 33 (97.0%) MM cases with the PCST method. No differences were noted between PCST and the conventional method in immunophenotyping and plasma cell percentages (P=0.323). Among plasma cells, levels (%) were significantly higher by the PCST approach than those in the conventional method (97.6% vs 95.8%, P=0.010). PCST exhibited better performance for plasma cell dyscrasias diagnosis, and could improve laboratory efficiency and quality.
Keyword
Figure
Reference
-
1. van Dongen JJ, Orfao A. 2012; EuroFlow: Resetting leukemia and lymphoma immunophenotyping. Basis for companion diagnostics and personalized medicine. Leukemia. 26:1899–907. DOI: 10.1038/leu.2012.121. PMID: 22948488. PMCID: PMC3437406.
Article2. van Dongen JJ, Lhermitte L, Böttcher S, Almeida J, van der Velden VH, Flores-Montero J, et al. 2012; EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 26:1908–75. DOI: 10.1038/leu.2012.120. PMID: 22552007. PMCID: PMC3437410.
Article3. Jeong TD, Park CJ, Shim H, Jang S, Chi HS, Yoon DH, et al. 2012; Simplified flow cytometric immunophenotyping panel for multiple myeloma, CD56/CD19/CD138(CD38)/CD45, to differentiate neoplastic myeloma cells from reactive plasma cells. Korean J Hematol. 47:260–6. DOI: 10.5045/kjh.2012.47.4.260. PMID: 23320004. PMCID: PMC3538797.
Article4. EuroFlow. EuroFlow standard operating protocol (SOP) for instrument setup and compensation, BC Navios Instruments. Version 1.4. https://www.euroflow.org/protocols. Updated on May 2014.5. Nadav L, Katz BZ, Baron S, Yossipov L, Polliack A, Deutsch V, et al. 2006; Diverse niches within multiple myeloma bone marrow aspirates affect plasma cell enumeration. Br J Haematol. 133:530–2. DOI: 10.1111/j.1365-2141.2006.06068.x. PMID: 16681641.
Article6. Cogbill CH, Spears MD, Vantuinen P, Harrington AM, Olteanu H, Kroft SH. 2015; Morphologic and cytogenetic variables affect theflow cytometric recovery of plasma cell myeloma cells in bone marrow aspirates. Int J Lab Hematol. 37:797–808. DOI: 10.1111/ijlh.12411. PMID: 26224420.7. Terstappen LW, Johnsen S, Segers-Nolten IM, Loken MR. 1990; Identification and characterization of plasma cells in normal human bone marrow by high-resolutionflow cytometry. Blood. 76:1739–47. DOI: 10.1182/blood.V76.9.1739.1739. PMID: 2224123.8. Leo R, Boeker M, Peest D, Hein R, Bartl R, Gessner JE, et al. 1992; Multiparameter analyses of normal and malignant human plasma cells: CD38++, CD56+, CD54+, cIg+ is the common phenotype of myeloma cells. Ann Hematol. 64:132–9. DOI: 10.1007/BF01697400. PMID: 1373957.
Article9. Harada H, Kawano MM, Huang N, Harada Y, Iwato K, Tanabe O, et al. 1993; Phenotypic difference of normal plasma cells from mature myeloma cells. Blood. 81:2658–63. DOI: 10.1182/blood.V81.10.2658.2658. PMID: 8490175.
Article10. Ruiz-Argüelles GJ, San Miguel JF. 1994; Cell surface markers in multiple myeloma. Mayo Clin Proc. 69:684–90. DOI: 10.1016/S0025-6196(12)61350-0. PMID: 8015335.11. Ocqueteau M, Orfao A, Almeida J, Bladé J, González M, García-Sanz R, et al. 1998; Immunophenotypic characterization of plasma cells from monoclonal gammopathy of undetermined significance patients. Implications for the differential diagnosis between MGUS and multiple myeloma. Am J Pathol. 152:1655–65.12. Lin P, Owens R, Tricot G, Wilson CS. 2004; Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 121:482–8. DOI: 10.1309/74R4TB90BUWH27JX. PMID: 15080299.
Article13. Seegmiller AC, Xu Y, McKenna RW, Karandikar NJ. 2007; Immunophenotypic differentiation between neoplastic plasma cells in mature B-cell lymphoma vs plasma cell myeloma. Am J Clin Pathol. 127:176–81. DOI: 10.1309/5EL22BH45PHUPM8P. PMID: 17210522.
Article14. Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, et al. 2008; Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 93:431–8. DOI: 10.3324/haematol.11080. PMID: 18268286.
Article15. Nowakowski GS, Witzig TE, Dingli D, Tracz MJ, Gertz MA, Lacy MQ, et al. 2005; Circulating plasma cells detected byflow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood. 106:2276–9. DOI: 10.1182/blood-2005-05-1858. PMID: 15961515. PMCID: PMC1895270.16. Gonsalves WI, Morice WG, Rajkumar V, Gupta V, Timm MM, Dispenzieri A, et al. 2014; Quantification of clonal circulating plasma cells in relapsed multiple myeloma. Br J Haematol. 167:500–5. DOI: 10.1111/bjh.13067. PMID: 25113422. PMCID: PMC4211997.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasma cell leukemia with rouleaux formation involving neoplastic cells and RBC
- A case of primary plasma cell leukemia exhibiting hemophagocytic plasma cells relapsed with multiple cutaneous plasmacytoma
- Long-Lived Plasma Cell
- Aberrant CD3 Expression in a Relapsed Plasma Cell Neoplasm
- A case of primary plasma cell leukemia