Restor Dent Endod.
2020 Nov;45(4):e52. 10.5395/rde.2020.45.e52.
Cytotoxicity and biocompatibility of high mol% yttria containing zirconia
- Affiliations
-
- 1Department of Biosystems Engineering, Graduate School of Science and Technology, Yamagata University, Yamagata, Japan
- KMID: 2512043
- DOI: http://doi.org/10.5395/rde.2020.45.e52
Abstract
Objectives
Yttria-stabilized tetragonal phase zirconia has been used as a dental restorative material for over a decade. While it is still the strongest and toughest ceramic, its translucency remains as a significant drawback. To overcome this, stabilizing the translucency zirconia to a significant cubic crystalline phase by increasing the yttria content to more than 8 mol% (8YTZP). However, the biocompatibility of a high amount of yttria is still an important topic that needs to be investigated.
Materials and Methods
Commercially available 8YTZP plates were used. To enhance cell adhesion, proliferation, and differentiation, the surface of the 8YTZP is sequentially polished with a SiC-coated abrasive paper and surface coating with type I collagen. Fibroblast-like cells L929 used for cell adherence and cell proliferation analysis, and mouse bone marrow-derived mesenchymal stem cells (BMSC) used for cell differentiation analysis.
Results
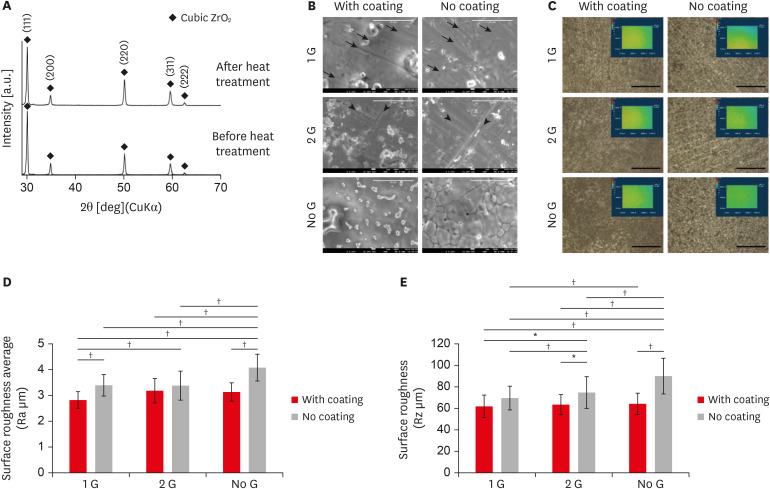
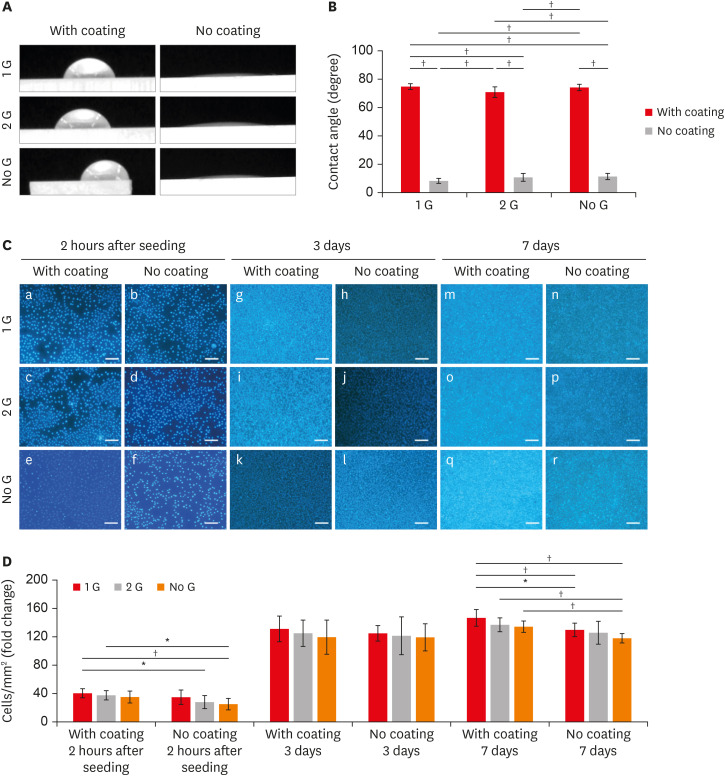
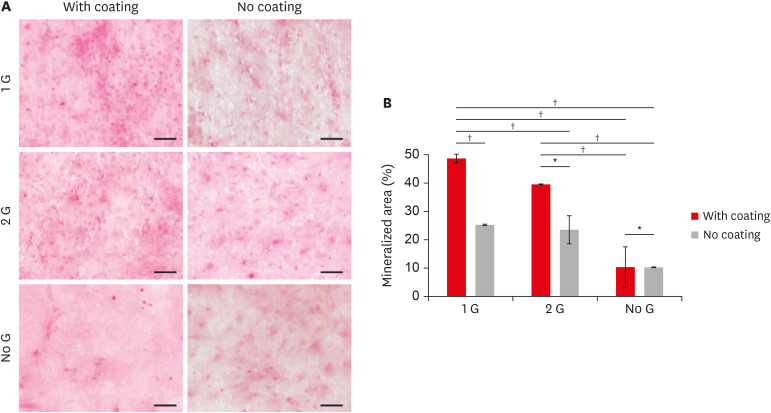
The results revealed that all samples, regardless of the surface treatment, are hydrophilic and showed a strong affinity for water. Even the cell culture results indicate that simple surface polishing and coating can affect cellular behavior by enhancing cell adhesion and proliferation. Both L929 cells and BMSC were nicely adhered to and proliferated in all conditions.
Conclusions
The results demonstrate the biocompatibility of the cubic phase zirconia with 8 mol% yttria and suggest that yttria with a higher zirconia content are not toxic to the cells, support a strong adhesion of cells on their surfaces, and promote cell proliferation and differentiation. All these confirm its potential use in tissue engineering.
Keyword
Figure
Reference
-
1. Abduo J, Lyons K, Swain M. Fit of zirconia fixed partial denture: a systematic review. J Oral Rehabil. 2010; 37:866–876. PMID: 20557435.
Article2. Yin L, Song XF, Song YL, Huang T, Li J. An overview of in vitro abrasive finishing and CAD/CAM of bioceramics in restorative dentistry. Int J Mach Tools Manuf. 2006; 46:1013–1026.
Article3. Miyazaki T, Nakamura T, Matsumura H, Ban S, Kobayashi T. Current status of zirconia restoration. J Prosthodont Res. 2013; 57:236–261. PMID: 24140561.
Article4. Chevalier J. What future for zirconia as a biomaterial? Biomaterials. 2006; 27:535–543. PMID: 16143387.
Article5. Zhang Y. Making yttria-stabilized tetragonal zirconia translucent. Dent Mater. 2014; 30:1195–1203. PMID: 25193781.
Article6. Zhang F, Inokoshi M, Batuk M, Hadermann J, Naert I, Van Meerbeek B, Vleugels J. Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent Mater. 2016; 32:e327–e337. PMID: 27697332.
Article7. Peuchert U, Okano Y, Menke Y, Reichel S, Ikesue A. Transparent cubic-ZrO2 ceramics for application as optical lenses. J Eur Ceram Soc. 2009; 29:283–291.8. Ang AS, Berndt CC. Investigating the anisotropic mechanical properties of plasma sprayed yttria-stabilised zirconia coatings. Surf Coat Technol. 2014; 259:551–559.
Article9. Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008; 29:2941–2953. PMID: 18440630.
Article10. Shin H, Ko H, Kim M. Cytotoxicity and biocompatibility of Zirconia (Y-TZP) posts with various dental cements. Restor Dent Endod. 2016; 41:167–175. PMID: 27508157.
Article11. Christel P, Meunier A, Heller M, Torre JP, Peille CN. Mechanical properties and short-term in-vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J Biomed Mater Res. 1989; 23:45–61. PMID: 2708404.
Article12. Ichikawa Y, Akagawa Y, Nikai H, Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo . J Prosthet Dent. 1992; 68:322–326. PMID: 1501183.13. Kannan SK, Sundrarajan M. Biosynthesis of Yttrium oxide nanoparticles using Acalypha indica leaf extract. Bull Mater Sci. 2015; 38:945–950.
Article14. Andelman T, Gordonov S, Busto G, Moghe PV, Riman RE. Synthesis and cytotoxicity of Y2O3 nanoparticles of various morphologies. Nanoscale Res Lett. 2009; 5:263–273. PMID: 20672046.15. Selvaraj V, Bodapati S, Murray E, Rice KM, Winston N, Shokuhfar T, Zhao Y, Blough E. Cytotoxicity and genotoxicity caused by yttrium oxide nanoparticles in HEK293 cells. Int J Nanomedicine. 2014; 9:1379–1391. PMID: 24648735.
Article16. Zhou G, Li Y, Ma Y, Liu Z, Cao L, Wand D, Liu S, Xu W, Wang W. Size-dependent cytotoxicity of yttrium oxide nanoparticles on primary osteoblasts in vitro . J Nanopart Res. 2016; 18:135.17. Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009; 4:102–106. PMID: 19131962.
Article18. Shah KM, Stern MM, Stern AR, Pathak JL, Bravenboer N, Bakker AD. Osteocyte isolation and culture methods. Bonekey Rep. 2016; 5:838. PMID: 27648260.
Article19. Vichi A, Louca C, Corciolani G, Ferrari M. Color related to ceramic and zirconia restorations: a review. Dent Mater. 2011; 27:97–108. PMID: 21122905.
Article20. Raptis NV, Michalakis KX, Hirayama H. Optical behavior of current ceramic systems. Int J Periodontics Restorative Dent. 2006; 26:31–41. PMID: 16515094.21. Denry I, Kelly JR. State of the art of zirconia for dental applications. Dent Mater. 2008; 24:299–307. PMID: 17659331.
Article22. Zhang Y, Lawn BR. Evaluating dental zirconia. Dent Mater. 2019; 35:15–23. PMID: 30172379.
Article23. Sulaiman TA, Abdulmajeed AA, Donovan TE, Vallittu PK, Närhi TO, Lassila LV. The effect of staining and vacuum sintering on optical and mechanical properties of partially and fully stabilized monolithic zirconia. Dent Mater J. 2015; 34:605–610. PMID: 26438983.
Article24. Denry I, Kelly JR. Emerging ceramic-based materials for dentistry. J Dent Res. 2014; 93:1235–1242. PMID: 25274751.
Article25. Watanabe H, Saito K, Kokubun K, Sasaki H, Yoshinari M. Change in surface properties of zirconia and initial attachment of osteoblastlike cells with hydrophilic treatment. Dent Mater J. 2012; 31:806–814. PMID: 23037844.
Article26. Ardlin BI. Transformation-toughened zirconia for dental inlays, crowns and bridges: chemical stability and effect of low-temperature aging on flexural strength and surface structure. Dent Mater. 2002; 18:590–595. PMID: 12385900.
Article27. Wu CC, Wei CK, Ho CC, Ding SJ. Enhanced hydrophilicity and biocompatibility of dental zirconia ceramics by oxygen plasma treatment. Materials (Basel). 2015; 8:684–699. PMID: 28787965.
Article28. Takamori ER, Cruz R, Gonçalvez F, Zanetti RV, Zanetti A, Granjeiro JM. Effect of roughness of zirconia and titanium on fibroblast adhesion. Artif Organs. 2008; 32:305–309. PMID: 18370945.
Article29. Han Y, Yan Y, Lu C, Zhang Y, Xu K. Bioactivity and osteoblast response of the micro-arc oxidized zirconia films. J Biomed Mater Res A. 2009; 88:117–127. PMID: 18260135.
Article30. Kim HW, Georgiou G, Knowles JC, Koh YH, Kim HE. Calcium phosphates and glass composite coatings on zirconia for enhanced biocompatibility. Biomaterials. 2004; 25:4203–4213. PMID: 15046910.
Article31. Pelaez-Vargas A, Gallego-Perez D, Magallanes-Perdomo M, Fernandes MH, Hansford DJ, De Aza AH, Pena P, Monteiro FJ. Isotropic micropatterned silica coatings on zirconia induce guided cell growth for dental implants. Dent Mater. 2011; 27:581–589. PMID: 21459429.
Article32. Somaiah C, Kumar A, Mawrie D, Sharma A, Patil SD, Bhattacharyya J, Swaminathan R, Jaganathan BG. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS One. 2015; 10:e0145068. PMID: 26661657.
Article33. Hsu CM, Sun YS, Huang HH. Enhanced cell response to zirconia surface immobilized with type I collagen. J Dent Res. 2019; 98:556–563. PMID: 30786812.
Article34. Wei J, Yoshinari M, Takemoto S, Hattori M, Kawada E, Liu B, Oda Y. Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability. J Biomed Mater Res B Appl Biomater. 2007; 81:66–75. PMID: 16924616.
Article35. Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005; 74:49–58. PMID: 15924300.
Article36. Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci. 2007; 32:698–725.
Article37. Eriksson C, Nygren H, Ohlson K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials. 2004; 25:4759–4766. PMID: 15120522.
Article38. Huang SC, Wu BC, Ding SJ. Stem cell differentiation-induced calcium silicate cement with bacteriostatic activity. J Mater Chem B Mater Biol Med. 2015; 3:570–580. PMID: 32262339.
Article39. Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000; 21:667–681. PMID: 10711964.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Review on factors affecting the optical properties of dental zirconia
- A review of biocompatibility of zirconia: In vitro experiment
- A review of biocompatibility of zirconia and bioactivity as a zirconia implant: In vivo experiment
- Cytotoxicity and biocompatibility of Zirconia (Y-TZP) posts with various dental cements
- Properties of translucent zirconia and lithium disilicate glass-ceramics: a literature review