J Korean Med Sci.
2021 Jan;36(4):e40. 10.3346/jkms.2021.36.e40.
Regional and Chronological Variation of Chemosensory Dysfunction in COVID-19: a Meta-Analysis
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Interdisciplinary Program of Medical Informatics, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2511637
- DOI: http://doi.org/10.3346/jkms.2021.36.e40
Abstract
- Background
Olfactory and gustatory dysfunction are frequently reported in patients with coronavirus disease 2019 (COVID-19). However, the reported prevalence of olfactory and/or gustatory dysfunction varies widely, and the reason for the inter-study differences is unclear. Hence, in this meta-analysis, we performed subgroup analyses to investigate the factors that contribute to the inter-study variability in the prevalence of olfactory and gustatory dysfunction.
Methods
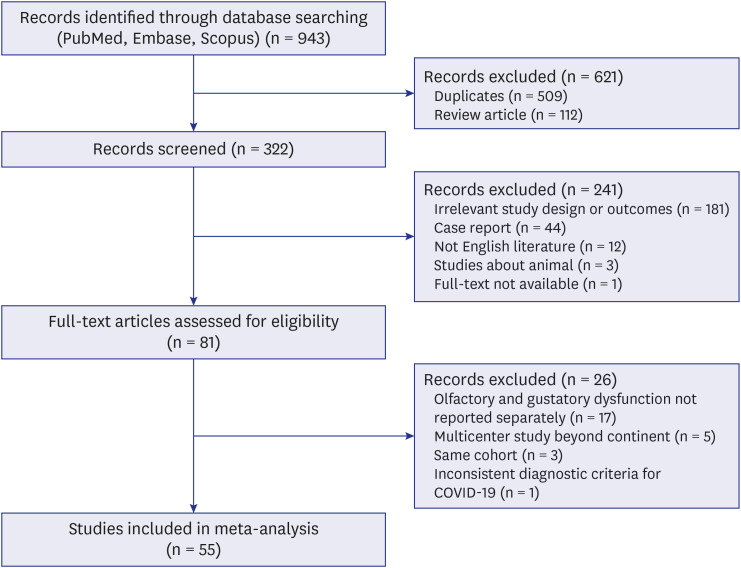
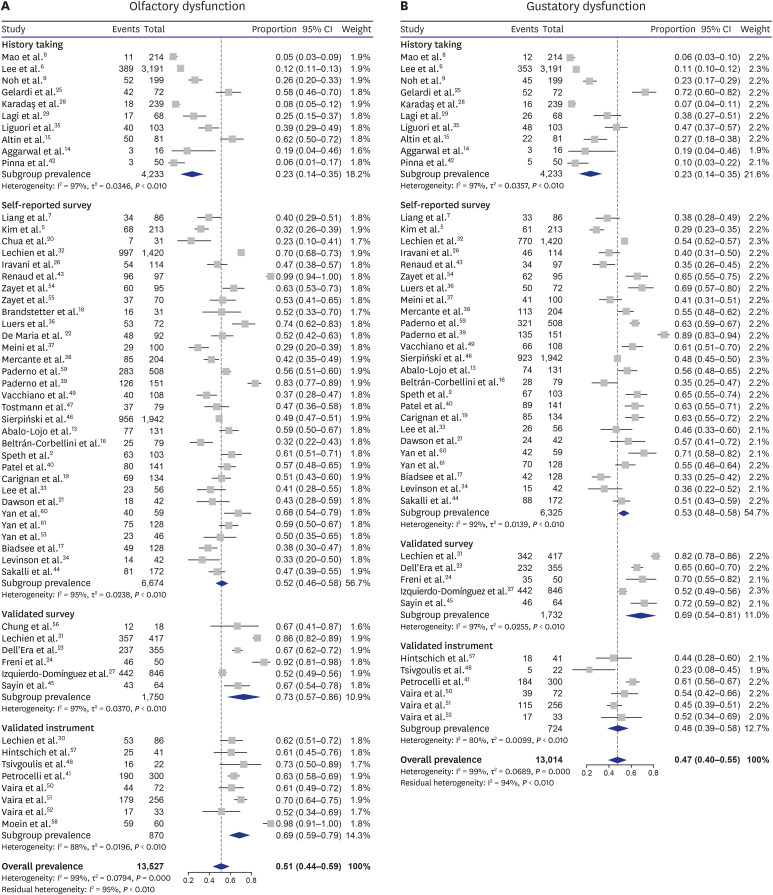
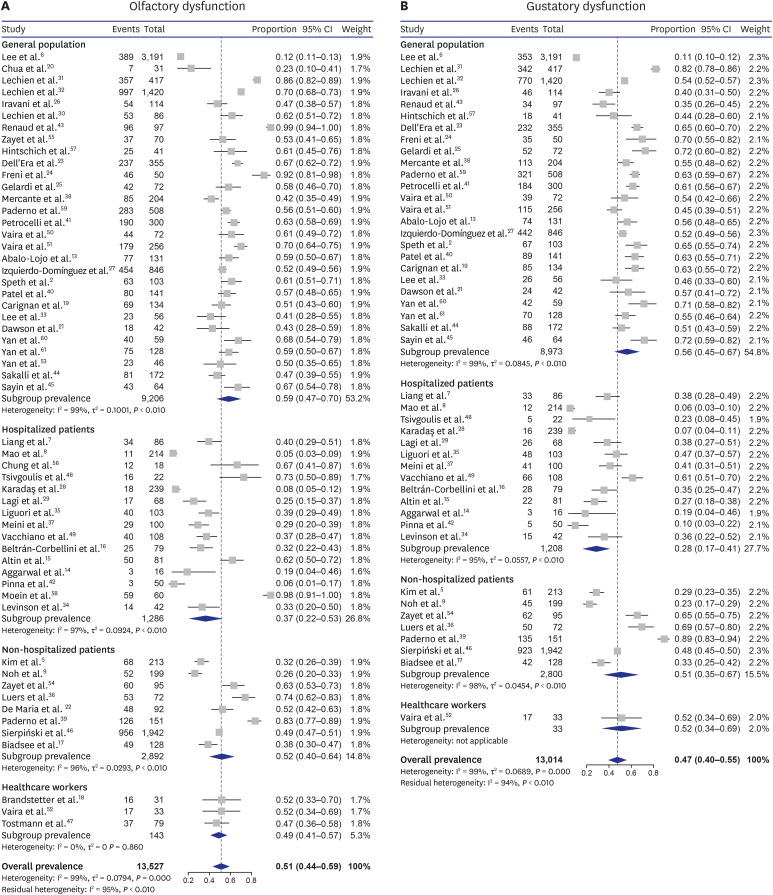
Out of 943 citations, we included 55 eligible studies with 13,527 patients with COVID-19 for a meta-analysis. Calculating the data extracted from each study, the weighted summary prevalence of olfactory and gustatory dysfunction was estimated using a FreemanTukey transformation with models based on random-effects assumptions. A meta-analysis of variance compared the prevalence of olfactory and gustatory dysfunction according to regional, chronological, demographic, and methodologic factors, respectively.
Results
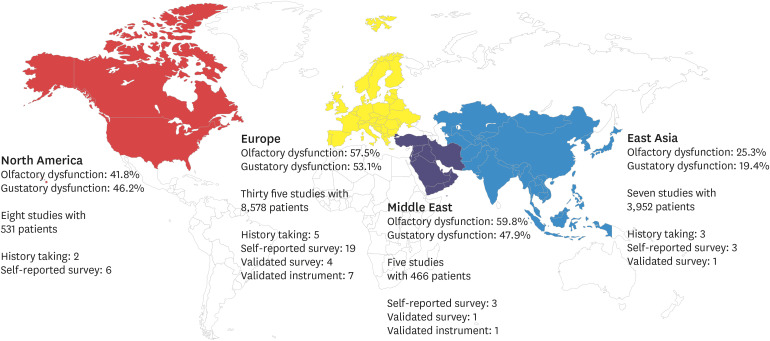
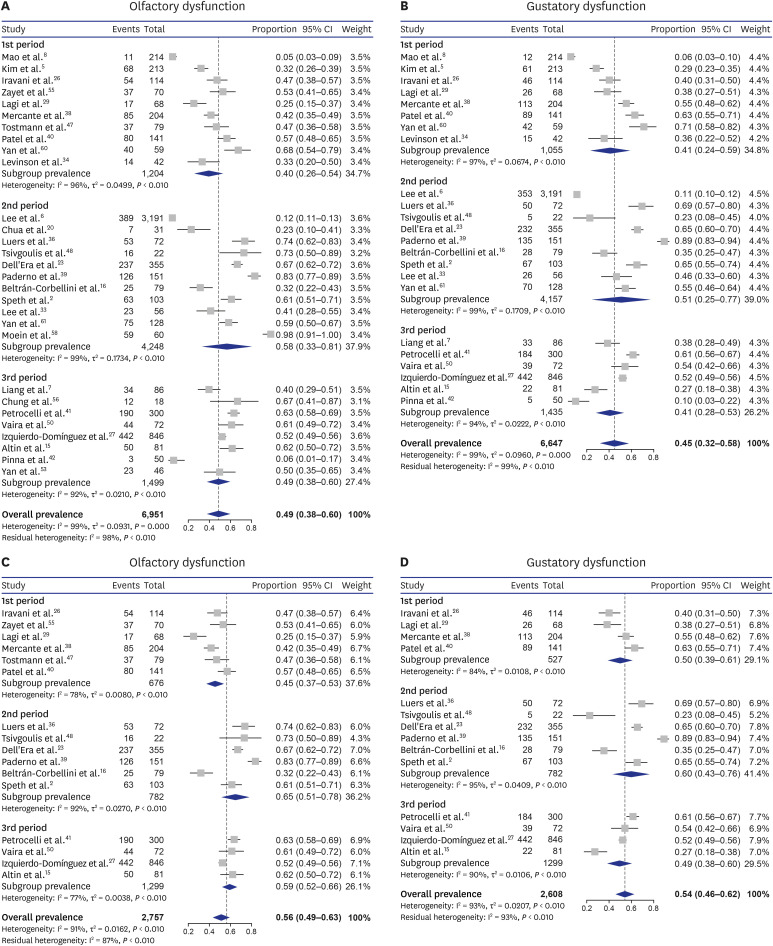
The overall pooled prevalence rates of olfactory and gustatory dysfunction were 51.4% and 47.5%, respectively, in the random-effect model. In subgroup analyses, the prevalence rates of olfactory and gustatory dysfunction were significantly different among four geographical regions (both P < 0.001, respectively). Although the prevalence rates of olfactory and gustatory dysfunction did not significantly differ according to the time of enrollment, the subgroup analyses including only studies from the same geographical region (Europe) revealed a significant difference in olfactory dysfunction according to the time of enrollment.
Conclusion
The regional and chronological differences in the prevalence rates of olfactory and gustatory dysfunctions partly explain the wide inter-study variability.
Keyword
Figure
Reference
-
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323(13):1239–1242. PMID: 32091533.2. Gengler I, Wang JC, Speth MM, Sedaghat AR. Sinonasal pathophysiology of SARS-CoV-2 and COVID-19: a systematic review of the current evidence. Laryngoscope Investig Otolaryngol. 2020; 5(3):354–359.3. Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020; 382(12):1177–1179. PMID: 32074444.
Article4. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020; 163(1):3–11. PMID: 32369429.
Article5. Kim GU, Kim MJ, Ra SH, Lee J, Bae S, Jung J, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020; 26(7):948.e1–948.e3. PMID: 32360780.
Article6. Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020; 35(18):e174. PMID: 32383370.
Article7. Liang Y, Xu J, Chu M, Mai J, Lai N, Tang W, et al. Neurosensory dysfunction: a diagnostic marker of early COVID-19. Int J Infect Dis. 2020; 98:347–352. PMID: 32615326.
Article8. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77(6):683–690. PMID: 32275288.
Article9. Noh JY, Yoon JG, Seong H, Choi WS, Sohn JW, Cheong HJ, et al. Asymptomatic infection and atypical manifestations of COVID-19: comparison of viral shedding duration. J Infect. 2020; 81(5):816–846.
Article10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62(10):e1–34. PMID: 19631507.
Article11. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012; 65(9):934–939. PMID: 22742910.
Article12. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950; 21(4):607–611.
Article13. Abalo-Lojo JM, Pouso-Diz JM, Gonzalez F. Taste and smell dysfunction in COVID-19 patients. Ann Otol Rhinol Laryngol. 2020; 129(10):1041–1042. PMID: 32468830.
Article14. Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis (Berl). 2020; 7(2):91–96. PMID: 32352401.
Article15. Altin F, Cingi C, Uzun T, Bal C. Olfactory and gustatory abnormalities in COVID-19 cases. Eur Arch Otorhinolaryngol. 2020; 277(10):2775–2781. PMID: 32577902.
Article16. Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez-Jorge F, Natera-Villalba E, Gómez-Corral J, et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020; 27(9):1738–1741. PMID: 32320508.
Article17. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms-a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020; 163(4):722–728. PMID: 32539587.
Article18. Brandstetter S, Roth S, Harner S, Buntrock-Döpke H, Toncheva AA, Borchers N, et al. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatr Allergy Immunol. 2020; 31(7):841–847. PMID: 32413201.
Article19. Carignan A, Valiquette L, Grenier C, Musonera JB, Nkengurutse D, Marcil-Héguy A, et al. Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. CMAJ. 2020; 192(26):E702–7. PMID: 32461325.
Article20. Chua AJ, Charn TC, Chan EC, Loh J. Acute olfactory loss is specific for COVID-19 at the emergency department. Ann Emerg Med. 2020; 76(4):550–551.
Article21. Dawson P, Rabold EM, Laws RL, Conners EE, Gharpure R, Yin S, et al. Loss of taste and smell as distinguishing symptoms of COVID-19. Clin Infect Dis. 2020; ciaa799. PMID: 32562541.
Article22. De Maria A, Varese P, Dentone C, Barisione E, Bassetti M. High prevalence of olfactory and taste disorder during SARS-CoV-2 infection in outpatients. J Med Virol. 2020; 92(11):2310–2311. PMID: 32383174.
Article23. Dell’Era V, Farri F, Garzaro G, Gatto M, Aluffi Valletti P, Garzaro M. Smell and taste disorders during COVID-19 outbreak: cross-sectional study on 355 patients. Head Neck. 2020; 42(7):1591–1596. PMID: 32524707.24. Freni F, Meduri A, Gazia F, Nicastro V, Galletti C, Aragona P, et al. Symptomatology in head and neck district in coronavirus disease (COVID-19): a possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol. 2020; 41(5):102612. PMID: 32574896.
Article25. Gelardi M, Trecca E, Cassano M, Ciprandi G. Smell and taste dysfunction during the COVID-19 outbreak: a preliminary report. Acta Biomed. 2020; 91(2):230–231. PMID: 32420954.26. Iravani B, Arshamian A, Ravia A, Mishor E, Snitz K, Shushan S, et al. Relationship between odor intensity estimates and COVID-19 prevalence prediction in a Swedish population. Chem Senses. Forthcoming. 2020.
Article27. Izquierdo-Domínguez A, Rojas-Lechuga M, Chiesa-Estomba C, Calvo-Henríquez C, Ninchritz-Becerra E, Soriano-Reixach M, et al. Smell and taste dysfunctions in COVID-19 are associated with younger age in ambulatory settings-a multicenter cross-sectional study. J Investig Allergol Clin Immunol. 2020; 30(5):346–357.28. Karadaş Ö, Öztürk B, Sonkaya AR. A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci. 2020; 41(8):1991–1995. PMID: 32588367.
Article29. Lagi F, Piccica M, Graziani L, Vellere I, Botta A, Tilli M, et al. Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Euro Surveill. 2020; 25(17):2000556.
Article30. Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M, Hans S, Calvo-Henriquez C, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020; 42(7):1583–1590. PMID: 32437033.
Article31. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; 277(8):2251–2261. PMID: 32253535.
Article32. Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020; 288(3):335–344. PMID: 32352202.33. Lee DJ, Lockwood J, Das P, Wang R, Grinspun E, Lee JM. Self-reported anosmia and dysgeusia as key symptoms of COVID-19. CJEM. 2020; 22(5):595–602. PMID: 32507123.34. Levinson R, Elbaz M, Ben-Ami R, Shasha D, Levinson T, Choshen G, et al. Time course of anosmia and dysgeusia in patients with mild SARS-CoV-2 infection. Infect Dis (Lond). 2020; 52(8):600–602. PMID: 32552475.
Article35. Liguori C, Pierantozzi M, Spanetta M, Sarmati L, Cesta N, Iannetta M, et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun. 2020; 88:11–16. PMID: 32416289.
Article36. Luers JC, Rokohl AC, Loreck N, Wawer Matos PA, Augustin M, Dewald F, et al. Olfactory and gustatory dysfunction in coronavirus disease 19 (COVID-19). Clin Infect Dis. 2020; 71(16):2262–2264. PMID: 32357210.37. Meini S, Suardi LR, Busoni M, Roberts AT, Fortini A. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. 2020; 277(12):3519–3523. PMID: 32500326.
Article38. Mercante G, Ferreli F, De Virgilio A, Gaino F, Di Bari M, Colombo G, et al. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020; 146(8):723.
Article39. Paderno A, Mattavelli D, Rampinelli V, Grammatica A, Raffetti E, Tomasoni M, et al. Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg. 2020; 163(6):1144–1149. PMID: 32600175.
Article40. Patel A, Charani E, Ariyanayagam D, Abdulaal A, Denny SJ, Mughal N, et al. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin Microbiol Infect. 2020; 26(9):1236–1241. PMID: 32502645.
Article41. Petrocelli M, Ruggiero F, Baietti AM, Pandolfi P, Salzano G, Salzano FA, et al. Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: the Bologna experience of 300 cases. J Laryngol Otol. 2020; 134(7):571–576. PMID: 32605666.
Article42. Pinna P, Grewal P, Hall JP, Tavarez T, Dafer RM, Garg R, et al. Neurological manifestations and COVID-19: experiences from a tertiary care center at the frontline. J Neurol Sci. 2020; 415:116969. PMID: 32570113.
Article43. Renaud M, Leon A, Trau G, Fath L, Ciftci S, Bensimon Y, et al. Acute smell and taste loss in outpatients: all infected with SARS-CoV-2? Rhinology. 2020; 58(4):406–409. PMID: 32542238.
Article44. Sakalli E, Temirbekov D, Bayri E, Alis EE, Erdurak SC, Bayraktaroglu M. Ear nose throat-related symptoms with a focus on loss of smell and/or taste in COVID-19 patients. Am J Otolaryngol. 2020; 41(6):102622. PMID: 32629147.
Article45. Sayin İ, Yaşar KK, Yazici ZM. Taste and smell impairment in COVID-19: an AAO-HNS anosmia reporting tool-based comparative study. Otolaryngol Head Neck Surg. 2020; 163(3):473–479. PMID: 32513096.
Article46. Sierpiński R, Pinkas J, Jankowski M, Zgliczyński W, Wierzba W, Gujski M, et al. Sex differences in the frequency of gastrointestinal symptoms and olfactory or taste disorders in 1942 nonhospitalized patients with coronavirus disease 2019 (COVID-19). Pol Arch Intern Med. 2020; 130(6):501–505. PMID: 32491298.47. Tostmann A, Bradley J, Bousema T, Yiek WK, Holwerda M, Bleeker-Rovers C, et al. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020; 25(16):2000508.
Article48. Tsivgoulis G, Fragkou PC, Delides A, Karofylakis E, Dimopoulou D, Sfikakis PP, et al. Quantitative evaluation of olfactory dysfunction in hospitalized patients with coronavirus [2] (COVID-19). J Neurol. 2020; 267(8):2193–2195. PMID: 32451613.
Article49. Vacchiano V, Riguzzi P, Volpi L, Tappatà M, Avoni P, Rizzo G, et al. Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci. 2020; 41(8):2029–2031. PMID: 32617738.
Article50. Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020; 42(6):1252–1258. PMID: 32342566.51. Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020; 42(7):1560–1569. PMID: 32437022.52. Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck. 2020; 42(7):1570–1576. PMID: 32357379.53. Yan CH, Prajapati DP, Ritter ML, DeConde AS. Persistent smell loss following undetectable SARS-CoV-2. Otolaryngol Head Neck Surg. 2020; 163(5):923–925. PMID: 32513065.
Article54. Zayet S, Klopfenstein T, Mercier J, Kadiane-Oussou NJ, Lan Cheong Wah L, Royer PY, et al. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection. Forthcoming. 2020.
Article55. Zayet S, Kadiane-Oussou NJ, Lepiller Q, Zahra H, Royer PY, Toko L, et al. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes Infect. 2020; 22(9):481–488. PMID: 32561409.
Article56. Chung TW, Sridhar S, Zhang AJ, Chan KH, Li HL, Wong FK, et al. Olfactory dysfunction in coronavirus disease 2019 patients: observational cohort study and systematic review. Open Forum Infect Dis. 2020; 7(6):a199.
Article57. Hintschich CA, Wenzel JJ, Hummel T, Hankir MK, Kühnel T, Vielsmeier V, et al. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int Forum Allergy Rhinol. 2020; 10(9):1105–1107. PMID: 32613712.
Article58. Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020; 10(8):944–950. PMID: 32301284.
Article59. Paderno A, Schreiber A, Grammatica A, Raffetti E, Tomasoni M, Gualtieri T, et al. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 2020; 10(8):955–962. PMID: 32410386.
Article60. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020; 10(7):806–813. PMID: 32279441.
Article61. Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020; 10(7):821–831. PMID: 32329222.
Article62. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020; 58(3):295–298. PMID: 32277751.
Article63. Han AY, Mukdad L, Long JL, Lopez IA. Anosmia in COVID-19: mechanisms and significance. Chem Senses. Forthcoming. 2020.
Article64. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020; 395(10224):565–574. PMID: 32007145.65. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579(7798):270–273. PMID: 32015507.66. Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020; 6(1):11. PMID: 32133153.
Article67. Hadfield J, Megill C, Bell SM, Huddleston J, Potter B, Callender C, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018; 34(23):4121–4123. PMID: 29790939.
Article68. Tang X, Wu C, Li X, Song Y, Yao X, Wu X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. 2020; 7(6):1012–1023.
Article69. Brufsky A. Distinct viral clades of SARS-CoV-2: implications for modeling of viral spread. J Med Virol. 2020; 92(9):1386–1390. PMID: 32311094.
Article70. Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020; 182(4):812–827.e19. PMID: 32697968.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Air Pollution on Chemosensory Dysfunction in COVID-19 Patients
- The Anxiety Status of Chinese Medical Workers During the Epidemic of COVID-19: A Meta-Analysis
- Olfactory and Taste Dysfunction in Patients with Asymptomatic and Mildly Symptomatic COVID-19 in Korea
- The Prevalence of Post-Traumatic Stress Disorder in the General Population during the COVID-19 Pandemic: A Systematic Review and Single-Arm Meta-Analysis
- Contemporary Review of Olfactory Dysfunction in COVID-19