Updates in the Pathologic Classification of Thyroid Neoplasms: A Review of the World Health Organization Classification
- Affiliations
-
- 1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Pathology, Peking University Cancer Hospital & Institute, Beijing, China
- 2Department of Pathology and Thyroid Disease Center, Izumi City General Hospital, Izumi, Japan
- 3Department of Human Pathology, Wakayama Medical University, Graduate School of Medicine, Wakayama, Japan
- 4Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2510998
- DOI: http://doi.org/10.3803/EnM.2020.807
Abstract
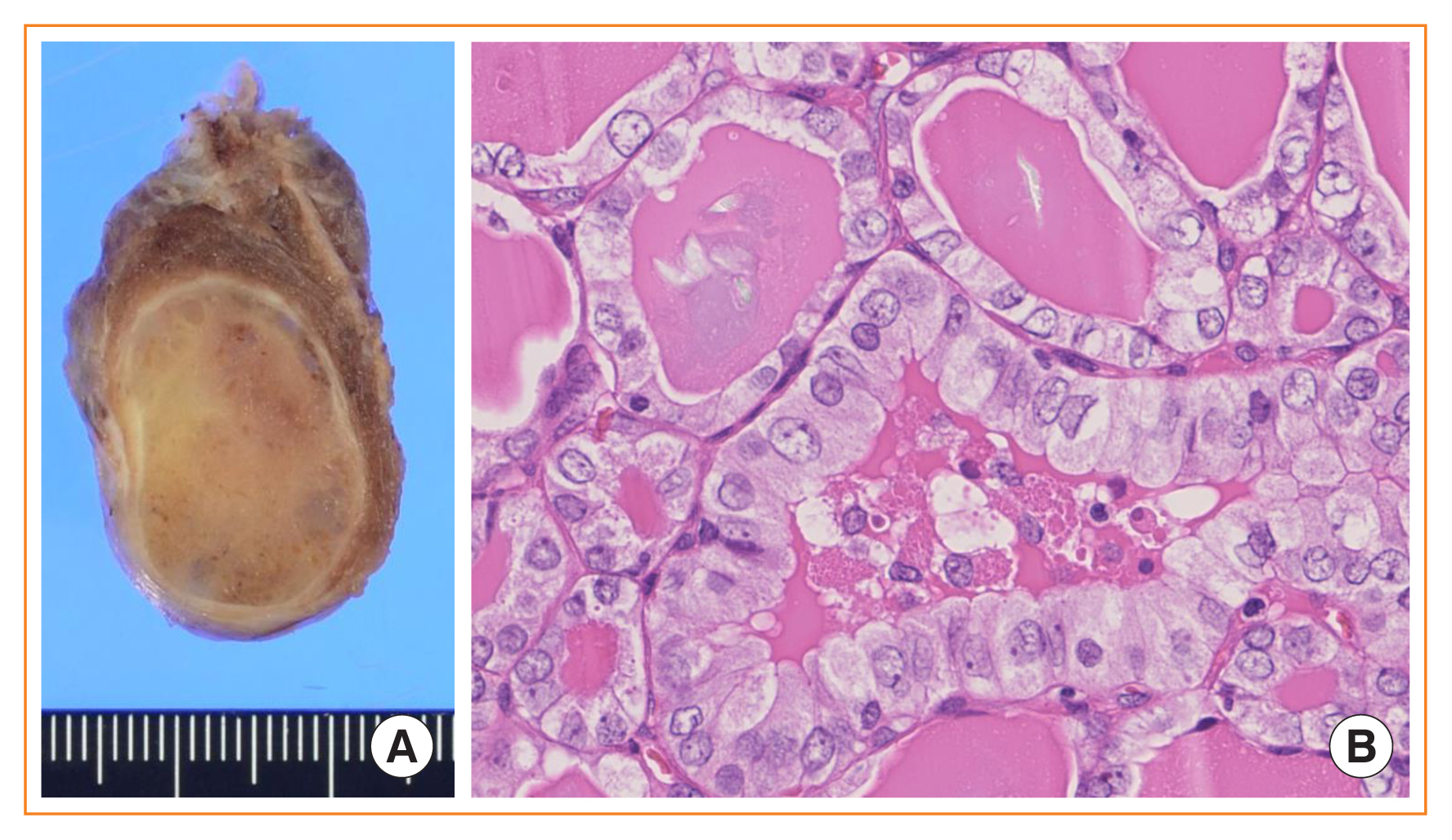
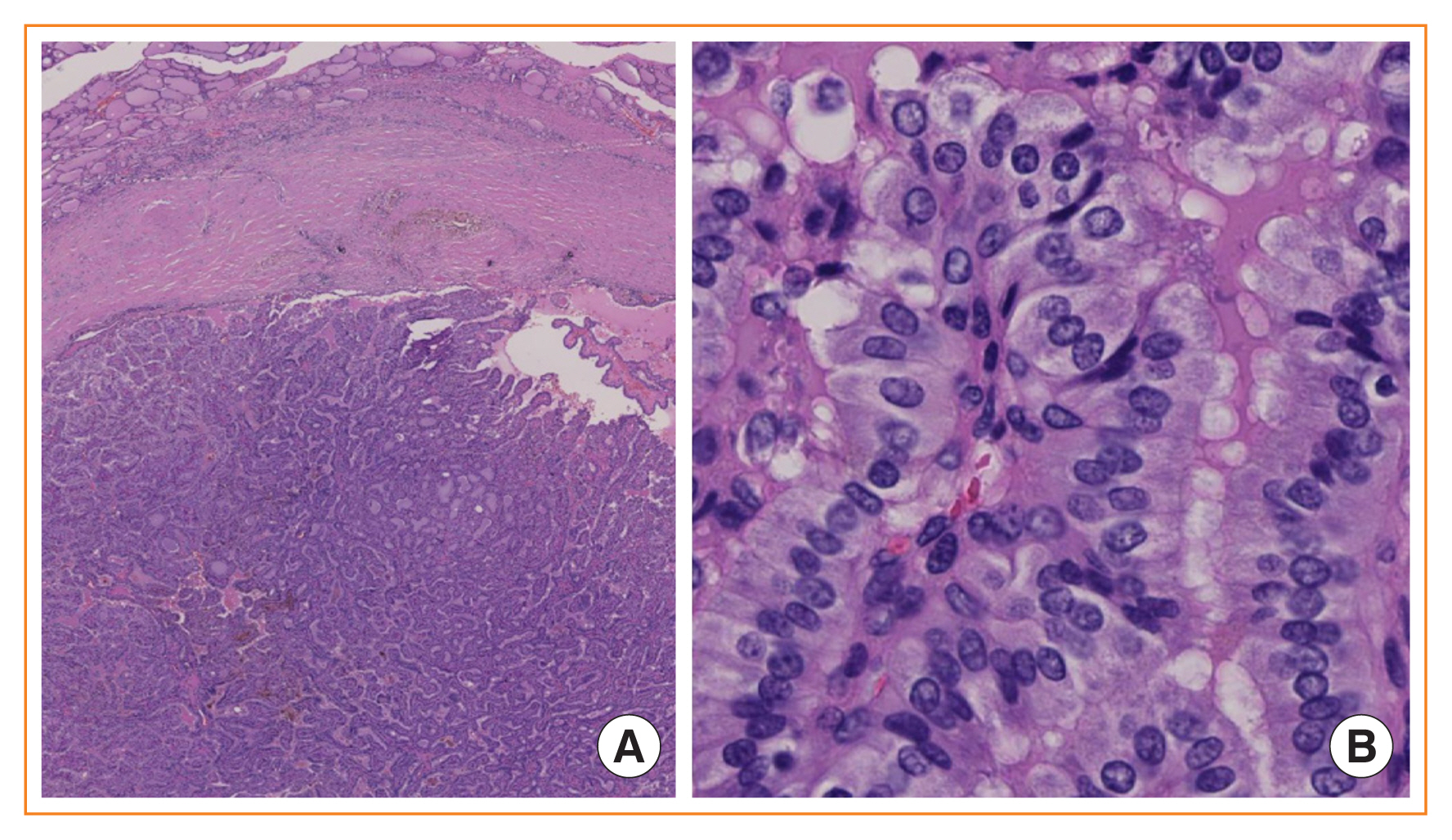
- Advances in medical sciences and evidence-based medicine have led to momentous changes in classification and management of thyroid neoplasms. Much progress has been made toward avoiding overdiagnosis and overtreatment of thyroid cancers. The new 2017 World Health Organization (WHO) classification of thyroid neoplasms updated the diagnostic criteria and molecular and genetic characteristics reflecting the biology and behavior of the tumors, and newly introduced the category of borderline malignancy or uncertain malignant potential. Some neoplasms were subclassified, renamed, or redefined as a specific entity. This review introduces changes in the fourth edition WHO classification of thyroid tumors and updates the contemporary diagnosis and classification of thyroid tumors. We also discuss several challenges with the proposal of new diagnostic entities, since they have unique histopathologic and molecular features and clinical relevance.
Figure
Cited by 4 articles
-
Best Achievements in Clinical Thyroidology in 2020
Eun Kyung Lee, Young Joo Park
Endocrinol Metab. 2021;36(1):30-35. doi: 10.3803/EnM.2021.103.Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach
Chan Kwon Jung, Andrey Bychkov, Kennichi Kakudo
Endocrinol Metab. 2022;37(5):703-718. doi: 10.3803/EnM.2022.1553.The Asian Thyroid Working Group, from 2017 to 2023
Kennichi Kakudo, Chan Kwon Jung, Zhiyan Liu, Mitsuyoshi Hirokawa, Andrey Bychkov, Huy Gia Vuong, Somboon Keelawat, Radhika Srinivasan, Jen-Fan Hang, Chiung-Ru Lai
J Pathol Transl Med. 2023;57(6):289-304. doi: 10.4132/jptm.2023.10.04.Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part I. Initial Management of Differentiated Thyroid Cancers - Chapter 4. Pathological Diagnosis and Staging after Thyroidectomy 2024
Su-Jin Shin, Hee Young Na, Ho-Cheol Kang, Sun Wook Kim, Dong Gyu Na, Young Joo Park, Young Shin Song, Eun Kyung Lee, Dong-Jun Lim, Yun Jae Chung, Chan Kwon Jung
Int J Thyroidol. 2024;17(1):61-67. doi: 10.11106/ijt.2024.17.1.61.
Reference
-
1. Delellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization classification of tumours of endocrine organs. 3rd ed. Lyon: International Agency for Research on Cancer (IARC);2004. p. 49–123.2. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer (IARC);2017. p. 65–143.3. Williams ED. Guest editorial: two proposals regarding the terminology of thyroid tumors. Int J Surg Pathol. 2000; 8:181–3.
Article4. Carney JA, Hirokawa M, Lloyd RV, Papotti M, Sebo TJ. Hyalinizing trabecular tumors of the thyroid gland are almost all benign. Am J Surg Pathol. 2008; 32:1877–89.
Article5. Kakudo K, Bai Y, Liu Z, Li Y, Ito Y, Ozaki T. Classification of thyroid follicular cell tumors: with special reference to borderline lesions. Endocr J. 2012; 59:1–12.
Article6. Kakudo K, Bai Y, Liu Z, Ozaki T. Encapsulated papillary thyroid carcinoma, follicular variant: a misnomer. Pathol Int. 2012; 62:155–60.
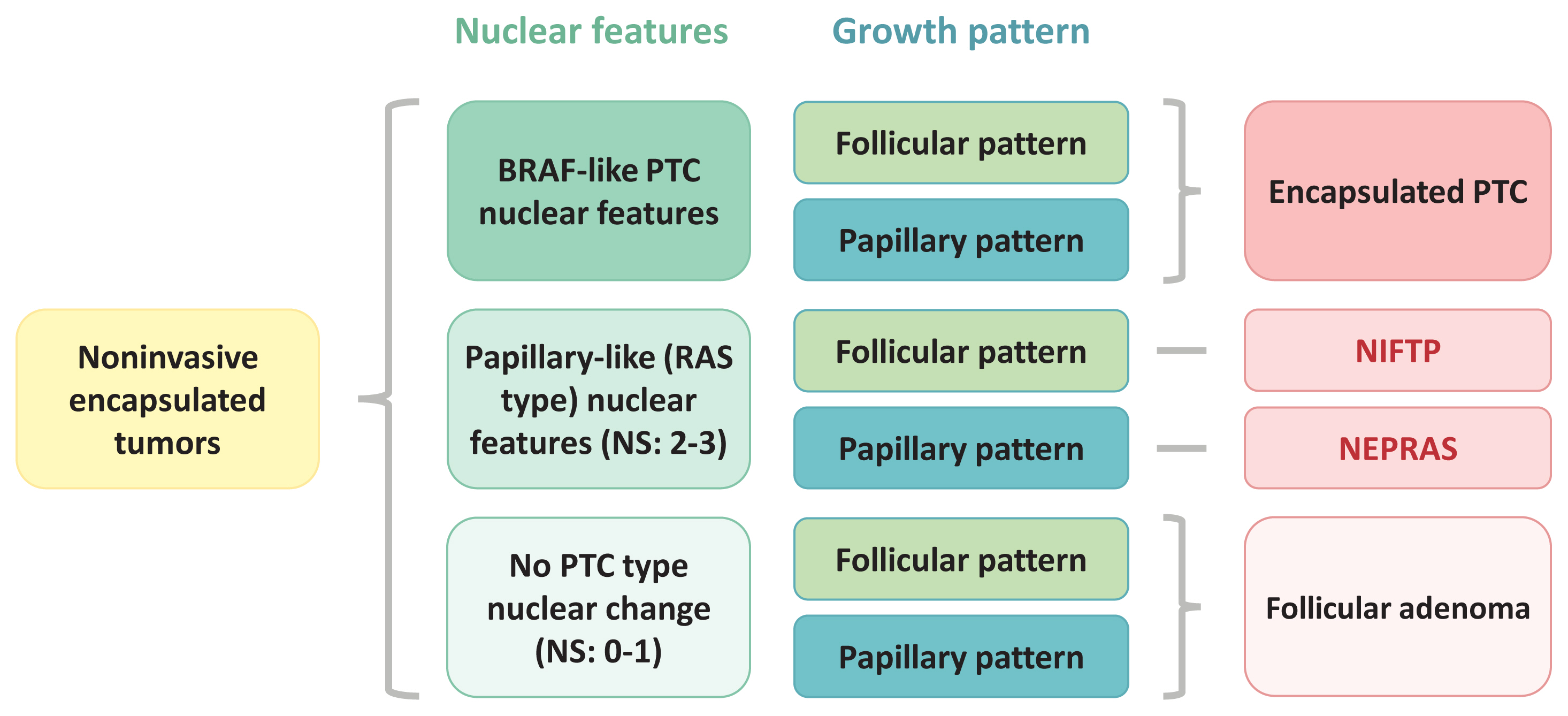
Article7. Kakudo K, El-Naggar AK, Hodak SP, Khanafshar E, Nikiforov YE, Nose V, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in thyroid tumor classification. Pathol Int. 2018; 68:327–33.
Article8. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–9.
Article9. Baloch ZW, Seethala RR, Faquin WC, Papotti MG, Basolo F, Fadda G, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a changing paradigm in thyroid surgical pathology and implications for thyroid cytopathology. Cancer Cytopathol. 2016; 124:616–20.
Article10. Bychkov A, Hirokawa M, Jung CK, Liu Z, Zhu Y, Hong SW, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid. 2017; 27:983–4.
Article11. Bychkov A, Keelawat S, Agarwal S, Jain D, Jung CK, Hong S, et al. Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: a multi-institutional study in five Asian countries. Pathology. 2018; 50:411–7.
Article12. Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol. 2017; 30:810–25.
Article13. Akaishi J, Kondo T, Sugino K, Ogimi Y, Masaki C, Hames KY, et al. Prognostic impact of the turin criteria in poorly differentiated thyroid carcinoma. World J Surg. 2019; 43:2235–44.
Article14. Ibrahim AA, Wu HH. Fine-needle aspiration cytology of noninvasive follicular variant of papillary thyroid carcinoma is cytomorphologically distinct from the invasive counterpart. Am J Clin Pathol. 2016; 146:373–7.
Article15. Maletta F, Massa F, Torregrossa L, Duregon E, Casadei GP, Basolo F, et al. Cytological features of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum Pathol. 2016; 54:134–42.
Article16. Parente DN, Kluijfhout WP, Bongers PJ, Verzijl R, Devon KM, Rotstein LE, et al. Clinical safety of renaming encapsulated follicular variant of papillary thyroid carcinoma: is NIFTP truly benign? World J Surg. 2018; 42:321–6.
Article17. Rosario PW, Mourao GF. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a review for clinicians. Endocr Relat Cancer. 2019; 26:R259–66.
Article18. Seethala RR, Baloch ZW, Barletta JA, Khanafshar E, Mete O, Sadow PM, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a review for pathologists. Mod Pathol. 2018; 31:39–55.
Article19. Strickland KC, Vivero M, Jo VY, Lowe AC, Hollowell M, Qian X, et al. Preoperative cytologic diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a prospective analysis. Thyroid. 2016; 26:1466–71.
Article20. Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to noninvasive follicular thyroid neoplasm with papillary-like nuclear features would help prevent overtreatment. Mod Pathol. 2016; 29:698–707.
Article21. Thompson LD, Poller DN, Kakudo K, Burchette R, Nikiforov YE, Seethala RR. An international interobserver variability reporting of the nuclear scoring criteria to diagnose noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a validation study. Endocr Pathol. 2018; 29:242–9.
Article22. Yang GC, Fried KO, Scognamiglio T. Sonographic and cytologic differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thyroid carcinoma: a review of 179 cases. Diagn Cytopathol. 2017; 45:533–41.23. Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002; 26:1508–14.
Article24. Liu Z, Bychkov A, Jung CK, Hirokawa M, Sui S, Hong S, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol Int. 2019; 69:202–10.
Article25. Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004; 28:1336–40.
Article26. Kakudo K, Liu Z, Bychkov A, Jung CK. Thyroid FNA cytology, differential diagnoses and pitfalls. 2nd ed. Singapore: Springer;2019. Chapter 21, Nuclear features of papillary thyroid carcinoma (BRAF-like tumors), noninvasive follicular thyroid neoplasm with papillary-like nuclear features (RAS-like tumors) and follicular adenoma/follicular thyroid carcinoma (RAS-like tumors). p. 173–9.27. Ohba K, Mitsutake N, Matsuse M, Rogounovitch T, Nishino N, Oki Y, et al. Encapsulated papillary thyroid tumor with delicate nuclear changes and a KRAS mutation as a possible novel subtype of borderline tumor. J Pathol Transl Med. 2019; 53:136–41.
Article28. Jung CK, Park SY, Kim JH, Kakudo K. New insights into classification and risk stratification of encapsulated thyroid tumors with a predominantly papillary architecture. J Pathol Transl Med. 2020; 54:197–203.
Article29. Rosario PW. Noninvasive encapsulated papillary RAS-like thyroid tumor (NEPRAS) or encapsulated papillary thyroid carcinoma (PTC). J Pathol Transl Med. 2020; 54:263–4.
Article30. Sambade C, Franssila K, Cameselle-Teijeiro J, Nesland J, Sobrinho-Simoes M. Hyalinizing trabecular adenoma: a misnomer for a peculiar tumor of the thyroid gland. Endocr Pathol. 1991; 2:83–91.
Article31. Nikiforova MN, Nikiforov YE, Ohori NP. GLIS rearrangements in thyroid nodules: a key to preoperative diagnosis of hyalinizing trabecular tumor. Cancer Cytopathol. 2019; 127:560–6.32. Marchio C, Da Cruz Paula A, Gularte-Merida R, Basili T, Brandes A, da Silva EM, et al. PAX8-GLIS3 gene fusion is a pathognomonic genetic alteration of hyalinizing trabecular tumors of the thyroid. Mod Pathol. 2019; 32:1734–43.
Article33. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.34. Nikiforova MN, Nikitski AV, Panebianco F, Kaya C, Yip L, Williams M, et al. GLIS rearrangement is a genomic hallmark of hyalinizing trabecular tumor of the thyroid gland. Thyroid. 2019; 29:161–73.
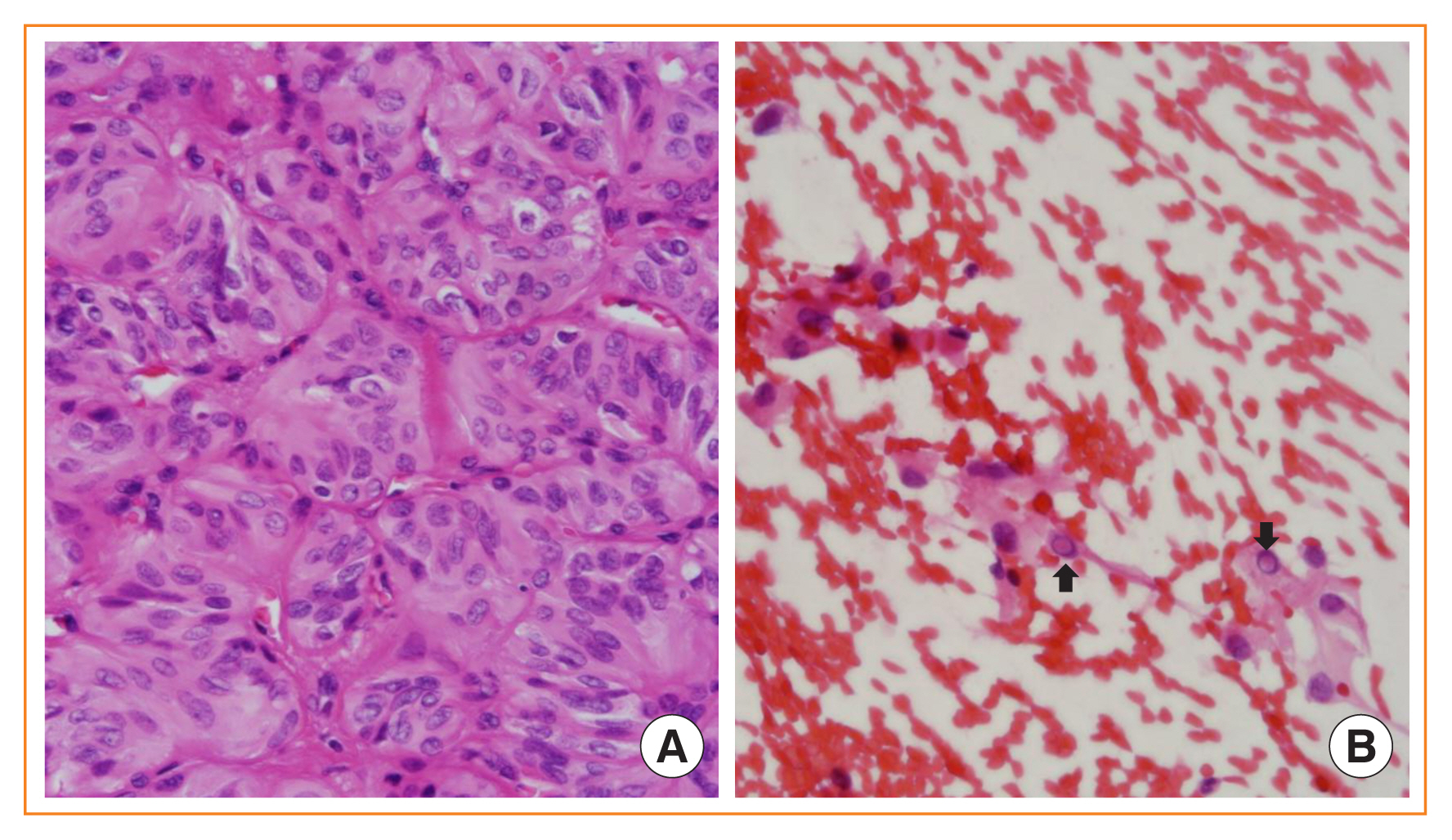
Article35. Nath MC, Erickson LA. Aggressive variants of papillary thyroid carcinoma: hobnail, tall cell, columnar, and solid. Adv Anat Pathol. 2018; 25:172–9.
Article36. Ho AS, Luu M, Barrios L, Chen I, Melany M, Ali N, et al. Incidence and mortality risk spectrum across aggressive variants of papillary thyroid carcinoma. JAMA Oncol. 2020; 6:706–13.
Article37. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article38. Song E, Jeon MJ, Oh HS, Han M, Lee YM, Kim TY, et al. Do aggressive variants of papillary thyroid carcinoma have worse clinical outcome than classic papillary thyroid carcinoma? Eur J Endocrinol. 2018; 179:135–42.
Article39. Limberg J, Ullmann TM, Stefanova D, Buicko JL, Finnerty BM, Zarnegar R, et al. Does aggressive variant histology without invasive features predict overall survival in papillary thyroid cancer?: a national cancer database analysis. Ann Surg. 2019. Oct. 9. [Epub]. https://doi.org/10.1097/SLA.00000-00000003632 .
Article40. Jung CK. Papillary thyroid carcinoma variants with tall columnar cells. J Pathol Transl Med. 2020; 54:123.
Article41. Ganly I, Ibrahimpasic T, Rivera M, Nixon I, Palmer F, Patel SG, et al. Prognostic implications of papillary thyroid carcinoma with tall-cell features. Thyroid. 2014; 24:662–70.
Article42. Wong KS, Higgins SE, Marqusee E, Nehs MA, Angell T, Barletta JA. Tall cell variant of papillary thyroid carcinoma: impact of change in WHO definition and molecular analysis. Endocr Pathol. 2019; 30:43–8.
Article43. Beninato T, Scognamiglio T, Kleiman DA, Uccelli A, Vaca D, Fahey TJ 3rd, et al. Ten percent tall cells confer the aggressive features of the tall cell variant of papillary thyroid carcinoma. Surgery. 2013; 154:1331–6.
Article44. Dettmer MS, Schmitt A, Steinert H, Capper D, Moch H, Komminoth P, et al. Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocr Relat Cancer. 2015; 22:419–29.
Article45. Vuong HG, Long NP, Anh NH, Nghi TD, Hieu MV, Hung LP, et al. Papillary thyroid carcinoma with tall cell features is as aggressive as tall cell variant: a meta-analysis. Endocr Connect. 2018; 7:R286–93.
Article46. Bongers PJ, Kluijfhout WP, Verzijl R, Lustgarten M, Vermeer M, Goldstein DP, et al. Papillary thyroid cancers with focal tall cell change are as aggressive as tall cell variants and should not be considered as low-risk disease. Ann Surg Oncol. 2019; 26:2533–9.
Article47. Oh WJ, Lee YS, Cho U, Bae JS, Lee S, Kim MH, et al. Classic papillary thyroid carcinoma with tall cell features and tall cell variant have similar clinicopathologic features. Korean J Pathol. 2014; 48:201–8.
Article48. Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer. 2008; 113:48–56.
Article49. Silver CE, Owen RP, Rodrigo JP, Rinaldo A, Devaney KO, Ferlito A. Aggressive variants of papillary thyroid carcinoma. Head Neck. 2011; 33:1052–9.
Article50. Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, et al. Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab. 2016; 101:264–74.
Article51. Villar-Taibo R, Peteiro-Gonzalez D, Cabezas-Agricola JM, Aliyev E, Barreiro-Morandeira F, Ruiz-Ponte C, et al. Aggressiveness of the tall cell variant of papillary thyroid carcinoma is independent of the tumor size and patient age. Oncol Lett. 2017; 13:3501–7.
Article52. Enriquez ML, Baloch ZW, Montone KT, Zhang PJ, LiVolsi VA. CDX2 expression in columnar cell variant of papillary thyroid carcinoma. Am J Clin Pathol. 2012; 137:722–6.
Article53. Sujoy V, Pinto A, Nose V. Columnar cell variant of papillary thyroid carcinoma: a study of 10 cases with emphasis on CDX2 expression. Thyroid. 2013; 23:714–9.
Article54. Yunta PJ, Ponce JL, Prieto M, Merino F, Sancho-Fornos S. The importance of a tumor capsule in columnar cell thyroid carcinoma: a report of two cases and review of the literature. Thyroid. 1999; 9:815–9.
Article55. Huang WT, Yang SF, Wang SL, Chan HM, Chai CY. Encapsulated columnar-cell carcinoma of the thyroid: a case report. Kaohsiung J Med Sci. 2005; 21:241–4.
Article56. Chen JH, Faquin WC, Lloyd RV, Nose V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod Pathol. 2011; 24:739–49.
Article57. Ieni A, Barresi V, Cardia R, Licata L, Di Bari F, Benvenga S, et al. The micropapillary/hobnail variant of papillary thyroid carcinoma: a review of series described in the literature compared to a series from one southern Italy pathology institution. Rev Endocr Metab Disord. 2016; 17:521–7.
Article58. Kakudo K, Tang W, Ito Y, Mori I, Nakamura Y, Miyauchi A. Papillary carcinoma of the thyroid in Japan: subclassification of common type and identification of low risk group. J Clin Pathol. 2004; 57:1041–6.
Article59. Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito Y, et al. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci. 2008; 99:1908–15.
Article60. Bai Y, Kakudo K, Nakamura M, Ozaki T, Li Y, Liu Z, et al. Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinoma and periostin expression. Cancer Lett. 2009; 281:188–95.
Article61. Asioli S, Erickson LA, Sebo TJ, Zhang J, Jin L, Thompson GB, et al. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma: a clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol. 2010; 34:44–52.
Article62. Liu Z, Kakudo K, Bai Y, Li Y, Ozaki T, Miyauchi A, et al. Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinoma, a novel predictor for lymph node metastasis; possible morphological indicator of epithelial mesenchymal transition. J Clin Pathol. 2011; 64:325–9.
Article63. Asioli S, Erickson LA, Righi A, Lloyd RV. Papillary thyroid carcinoma with hobnail features: histopathologic criteria to predict aggressive behavior. Hum Pathol. 2013; 44:320–8.
Article64. Ambrosi F, Righi A, Ricci C, Erickson LA, Lloyd RV, Asioli S. Hobnail variant of papillary thyroid carcinoma: a literature review. Endocr Pathol. 2017; 28:293–301.
Article65. Lee YS, Kim Y, Jeon S, Bae JS, Jung SL, Jung CK. Cytologic, clinicopathologic, and molecular features of papillary thyroid carcinoma with prominent hobnail features: 10 case reports and systematic literature review. Int J Clin Exp Pathol. 2015; 8:7988–97.66. Teng L, Deng W, Lu J, Zhang J, Ren X, Duan H, et al. Hobnail variant of papillary thyroid carcinoma: molecular profiling and comparison to classical papillary thyroid carcinoma, poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma. Oncotarget. 2017; 8:22023–33.
Article67. Lubitz CC, Economopoulos KP, Pawlak AC, Lynch K, Dias-Santagata D, Faquin WC, et al. Hobnail variant of papillary thyroid carcinoma: an institutional case series and molecular profile. Thyroid. 2014; 24:958–65.
Article68. Wong KS, Chen TY, Higgins SE, Howitt BE, Lorch JH, Alexander EK, et al. A potential diagnostic pitfall for hobnail variant of papillary thyroid carcinoma. Histopathology. 2020; 76:707–13.
Article69. Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007; 31:1256–64.
Article70. Collini P, Mattavelli F, Pellegrinelli A, Barisella M, Ferrari A, Massimino M. Papillary carcinoma of the thyroid gland of childhood and adolescence: morphologic subtypes, biologic behavior and prognosis: a clinicopathologic study of 42 sporadic cases treated at a single institution during a 30-year period. Am J Surg Pathol. 2006; 30:1420–6.71. LiVolsi VA, Abrosimov AA, Bogdanova T, Fadda G, Hunt JL, Ito M, et al. The Chernobyl thyroid cancer experience: pathology. Clin Oncol (R Coll Radiol). 2011; 23:261–7.
Article72. Nikiforov YE, Erickson LA, Nikiforova MN, Caudill CM, Lloyd RV. Solid variant of papillary thyroid carcinoma: incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior. Am J Surg Pathol. 2001; 25:1478–84.73. Tronko MD, Bogdanova TI, Komissarenko IV, Epstein OV, Oliynyk V, Kovalenko A, et al. Thyroid carcinoma in children and adolescents in Ukraine after the Chernobyl nuclear accident: statistical data and clinicomorphologic characteristics. Cancer. 1999; 86:149–56.
Article74. Ohashi R. Solid variant of papillary thyroid carcinoma: an under-recognized entity. Endocr J. 2020; 67:241–8.
Article75. Ohashi R, Kawahara K, Namimatsu S, Igarashi T, Sakatani T, Sugitani I, et al. Clinicopathological significance of a solid component in papillary thyroid carcinoma. Histopathology. 2017; 70:775–81.
Article76. Caplan RH, Wester S, Kisken AW. Diffuse sclerosing variant of papillary thyroid carcinoma: case report and review of the literature. Endocr Pract. 1997; 3:287–92.
Article77. Joung JY, Kim TH, Jeong DJ, Park SM, Cho YY, Jang HW, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: major genetic alterations and prognostic implications. Histopathology. 2016; 69:45–53.
Article78. Sheu SY, Schwertheim S, Worm K, Grabellus F, Schmid KW. Diffuse sclerosing variant of papillary thyroid carcinoma: lack of BRAF mutation but occurrence of RET/PTC rearrangements. Mod Pathol. 2007; 20:779–87.
Article79. Pillai S, Gopalan V, Smith RA, Lam AK. Diffuse sclerosing variant of papillary thyroid carcinoma: an update of its clinicopathological features and molecular biology. Crit Rev Oncol Hematol. 2015; 94:64–73.
Article80. Thompson LD, Wieneke JA, Heffess CS. Diffuse sclerosing variant of papillary thyroid carcinoma: a clinicopathologic and immunophenotypic analysis of 22 cases. Endocr Pathol. 2005; 16:331–48.
Article81. Koo JS, Hong S, Park CS. Diffuse sclerosing variant is a major subtype of papillary thyroid carcinoma in the young. Thyroid. 2009; 19:1225–31.
Article82. Fukushima M, Ito Y, Hirokawa M, Akasu H, Shimizu K, Miyauchi A. Clinicopathologic characteristics and prognosis of diffuse sclerosing variant of papillary thyroid carcinoma in Japan: an 18-year experience at a single institution. World J Surg. 2009; 33:958–62.
Article83. Malandrino P, Russo M, Regalbuto C, Pellegriti G, Moleti M, Caff A, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: a meta-analysis. Thyroid. 2016; 26:1285–92.
Article84. Kim HJ, Sung JY, Oh YL, Kim JH, Son YI, Min YK, et al. Association of vascular invasion with increased mortality in patients with minimally invasive follicular thyroid carcinoma but not widely invasive follicular thyroid carcinoma. Head Neck. 2014; 36:1695–700.
Article85. Xu B, Ghossein R. Encapsulated thyroid carcinoma of follicular cell origin. Endocr Pathol. 2015; 26:191–9.
Article86. O’Neill CJ, Vaughan L, Learoyd DL, Sidhu SB, Delbridge LW, Sywak MS. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur J Surg Oncol. 2011; 37:181–5.
Article87. Cracolici V, Ritterhouse LL, Segal JP, Puranik R, Wanjari P, Kadri S, et al. Follicular thyroid neoplasms: comparison of clinicopathologic and molecular features of atypical adenomas and follicular thyroid carcinomas. Am J Surg Pathol. 2020; 44:881–92.88. Maximo V, Sobrinho-Simoes M. Mitochondrial DNA ‘common’ deletion in Hurthle cell lesions of the thyroid. J Pathol. 2000; 192:561–2.89. Bishop JA, Wu G, Tufano RP, Westra WH. Histological patterns of locoregional recurrence in Hürthle cell carcinoma of the thyroid gland. Thyroid. 2012; 22:690–4.
Article90. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf). 2005; 63:87–93.
Article91. Gasparre G, Porcelli AM, Bonora E, Pennisi LF, Toller M, Iommarini L, et al. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc Natl Acad Sci U S A. 2007; 104:9001–6.
Article92. Maximo V, Soares P, Lima J, Cameselle-Teijeiro J, Sobrinho-Simoes M. Mitochondrial DNA somatic mutations (point mutations and large deletions) and mitochondrial DNA variants in human thyroid pathology: a study with emphasis on Hurthle cell tumors. Am J Pathol. 2002; 160:1857–65.93. Chindris AM, Casler JD, Bernet VJ, Rivera M, Thomas C, Kachergus JM, et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab. 2015; 100:55–62.
Article94. Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, et al. Integrated genomic analysis of Hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell. 2018; 34:256–70.
Article95. Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS. Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors. Hum Pathol. 2018; 81:9–17.
Article96. Sakamoto A, Kasai N, Sugano H. Poorly differentiated carcinoma of the thyroid: a clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer. 1983; 52:1849–55.
Article97. Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006; 106:1286–95.
Article98. Asioli S, Erickson LA, Righi A, Jin L, Volante M, Jenkins S, et al. Poorly differentiated carcinoma of the thyroid: validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol. 2010; 23:1269–78.
Article99. Bai S, Baloch ZW, Samulski TD, Montone KT, LiVolsi VA. Poorly differentiated oncocytic (Hürthle cell) follicular carcinoma: an institutional experience. Endocr Pathol. 2015; 26:164–9.
Article100. Ziad el A, Ruchala M, Breborowicz J, Gembicki M, Sowinski J, Grzymislawski M. Immunoexpression of TTF-1 and Ki-67 in a coexistent anaplastic and follicular thyroid cancer with rare long-life surviving. Folia Histochem Cytobiol. 2008; 46:461–4.101. Kakudo K, Wakasa T, Ohta Y, Yane K, Ito Y, Yamashita H. Prognostic classification of thyroid follicular cell tumors using Ki-67 labeling index: risk stratification of thyroid follicular cell carcinomas. Endocr J. 2015; 62:1–12.
Article102. Deeken-Draisey A, Yang GY, Gao J, Alexiev BA. Anaplastic thyroid carcinoma: an epidemiologic, histologic, immunohistochemical, and molecular single-institution study. Hum Pathol. 2018; 82:140–8.
Article103. Dettmer M, Schmitt A, Steinert H, Haldemann A, Meili A, Moch H, et al. Poorly differentiated thyroid carcinomas: how much poorly differentiated is needed? Am J Surg Pathol. 2011; 35:1866–72.104. Wong KS, Lorch JH, Alexander EK, Marqusee E, Cho NL, Nehs MA, et al. Prognostic significance of extent of invasion in poorly differentiated thyroid carcinoma. Thyroid. 2019; 29:1255–61.
Article105. Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly differentiated carcinoma of the thyroid gland: current status and future prospects. Thyroid. 2019; 29:311–21.
Article106. Bournaud C, Descotes F, Decaussin-Petrucci M, Berthiller J, de la Fouchardiere C, Giraudet AL, et al. TERT promoter mutations identify a high-risk group in metastasis-free advanced thyroid carcinoma. Eur J Cancer. 2019; 108:41–9.
Article107. Xu B, Ghossein R. Poorly differentiated thyroid carcinoma. Semin Diagn Pathol. 2020; 37:243–7.
Article108. Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg. 2015; 4:44–51.109. Kuhn E, Ragazzi M, Ciarrocchi A, Torricelli F, de Biase D, Zanetti E, et al. Angiosarcoma and anaplastic carcinoma of the thyroid are two distinct entities: a morphologic, immunohistochemical, and genetic study. Mod Pathol. 2019; 32:787–98.
Article110. Talbott I, Wakely PE Jr. Undifferentiated (anaplastic) thyroid carcinoma: practical immunohistochemistry and cytologic look-alikes. Semin Diagn Pathol. 2015; 32:305–10.
Article111. Bishop JA, Sharma R, Westra WH. PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum Pathol. 2011; 42:1873–7.
Article112. Lai WA, Hang JF, Liu CY, Bai Y, Liu Z, Gu H, et al. PAX8 expression in anaplastic thyroid carcinoma is less than those reported in early studies: a multi-institutional study of 182 cases using the monoclonal antibody MRQ-50. Virchows Arch. 2020; 476:431–7.
Article113. Nakazawa T, Kondo T, Vuong HG, Odate T, Kawai M, Tahara I, et al. High expression of CD10 in anaplastic thyroid carcinomas. Histopathology. 2018; 73:492–9.
Article114. Oh EJ, Bychkov A, Cho H, Kim TM, Bae JS, Lim DJ, et al. Prognostic implications of CD10 and CD15 expression in papillary thyroid carcinoma. Cancers (Basel). 2020; 12:1413.
Article115. Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, et al. Dissecting anaplastic thyroid carcinoma: a comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid. 2020; 30:1505–17.
Article116. Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014; 120:799–807.
Article117. Dogan S, Wang L, Ptashkin RN, Dawson RR, Shah JP, Sherman EJ, et al. Mammary analog secretory carcinoma of the thyroid gland: a primary thyroid adenocarcinoma harboring ETV6-NTRK3 fusion. Mod Pathol. 2016; 29:985–95.
Article118. Stevens TM, Kovalovsky AO, Velosa C, Shi Q, Dai Q, Owen RP, et al. Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative study. Mod Pathol. 2015; 28:1084–100.
Article119. Tirro E, Martorana F, Romano C, Vitale SR, Motta G, Di Gregorio S, et al. Molecular alterations in thyroid cancer: from bench to clinical practice. Genes (Basel). 2019; 10:709.
Article120. Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018; 15:731–47.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoplastic Diseases of the Hematopoietic and Lymphoid Tissues: New World Health Organization Classification
- Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach
- Understanding Neoplasm of Uncertain or Unknown Behavior of the Thyroid in Korean Clinical Practice
- Major Changes to the 2017 Revision of the World Health Organization Classification of Myeloproliferative Neoplasms
- What’s new in neuropathology 2024: CNS WHO 5th edition updates