Korean Circ J.
2021 Jan;51(1):68-80. 10.4070/kcj.2020.0117.
Nlrp3, Csf3, and Edn1 in Macrophage Response to Saturated Fatty Acids and Modified Low-Density Lipoprotein
- Affiliations
-
- 1Graduate Program of Science for Aging, Graduate School of Yonsei University, Seoul, Korea
- 2Division of Cardiology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2509906
- DOI: http://doi.org/10.4070/kcj.2020.0117
Abstract
- Background and Objectives
The relationship between metabolic stress, inflammation, and cardiovascular disease is being studied steadily. The aim of this study was to evaluate the effect of palmitate (PA) and minimally modified low-density lipoprotein (mmLDL) on macrophages and to identify the associated pathways.
Methods
J774 macrophages were incubated with PA or mmLDL and lipopolysaccharide (LPS). Secretion of inflammatory chemokines and the expression of corresponding genes were determined. The phosphorylation of extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase was also assessed. RNA sequencing of macrophages was performed to identify the genes regulated by PA or mmLDL. Some of the genes regulated by the 2 agents were validated by knocking down the cells using small interfering RNA.
Results
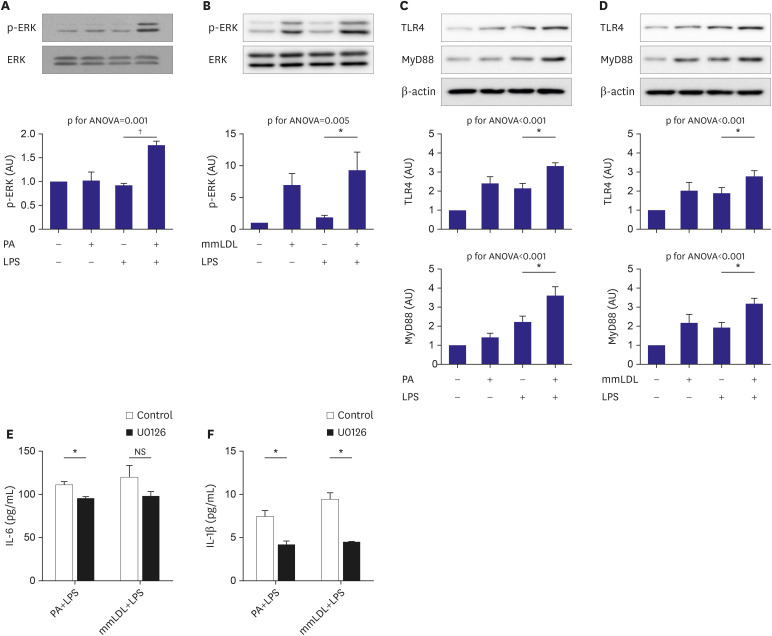
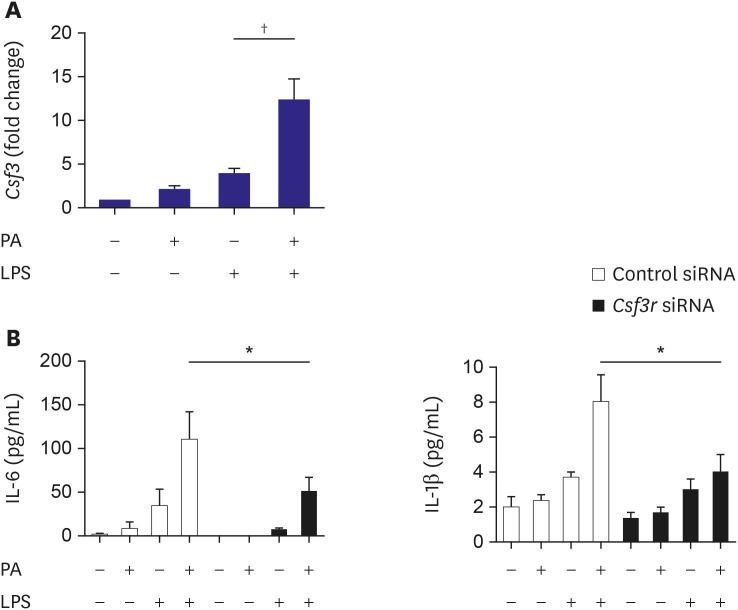
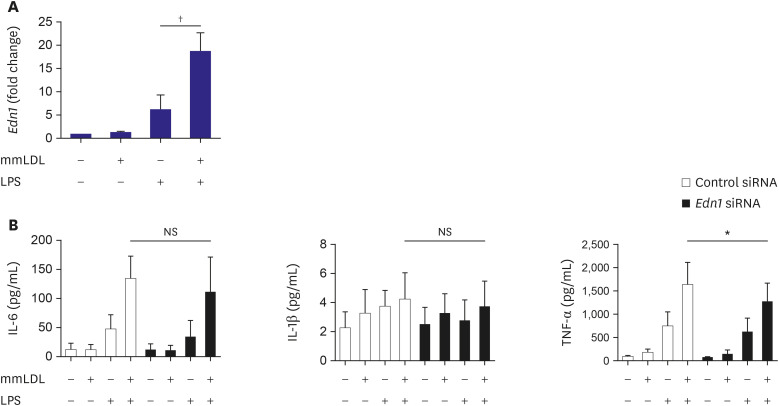
PA or mmLDL promoted the secretion of interleukin (IL)-6 and IL-1β in LPSstimulated macrophages, and this was accompanied by higher phosphorylation of ERK. RNA sequencing revealed dozens of genes that were regulated in this process, such as Csf3 and Edn1, which were affected by PA and mmLDL, respectively. These agents also increased Nlrp3 expression. The effect of Csf3 or Edn1 silencing on inflammation was modest, whereas toll-like receptor (TLR) 4 inhibition reduced a large proportion of macrophage activation.
Conclusions
We demonstrated that the proinflammatory milieu with high levels of PA or mmLDL promoted macrophage activation and the expression of associated genes such as Nlrp3, Csf3, and Edn1. Although the TLR4 pathway appeared to be most relevant, additional role of other genes in this process provided insights regarding the potential targets for intervention.
Keyword
Figure
Reference
-
1. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018; 34:575–584. PMID: 29459239.
Article2. Ali L, Schnitzler JG, Kroon J. Metabolism: the road to inflammation and atherosclerosis. Curr Opin Lipidol. 2018; 29:474–480. PMID: 30234554.3. Bettencourt IA, Powell JD. Targeting metabolism as a novel therapeutic approach to autoimmunity, inflammation, and transplantation. J Immunol. 2017; 198:999–1005. PMID: 28115589.
Article4. Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. 2016; 244:211–215. PMID: 26687466.
Article5. Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012; 15:518–533. PMID: 22465073.
Article6. Chung JH, Jeon HJ, Hong SY, et al. Palmitate promotes the paracrine effects of macrophages on vascular smooth muscle cells: the role of bone morphogenetic proteins. PLoS One. 2012; 7:e29100. PMID: 22363399.
Article7. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377:1119–1131. PMID: 28845751.8. Ann SJ, Kim KK, Cheon EJ, et al. Palmitate and minimally-modified low-density lipoprotein cooperatively promote inflammatory responses in macrophages. PLoS One. 2018; 13:e0193649. PMID: 29518116.
Article9. Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005; 25:1213–1219. PMID: 15718493.
Article10. Choi SH, Yin H, Ravandi A, et al. Polyoxygenated cholesterol ester hydroperoxide activates TLR4 and SYK dependent signaling in macrophages. PLoS One. 2013; 8:e83145. PMID: 24376657.
Article11. Schwartz EA, Zhang WY, Karnik SK, et al. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol. 2010; 30:802–808. PMID: 20110572.12. Sharma M, Boytard L, Hadi T, et al. Enhanced glycolysis and HIF-1α activation in adipose tissue macrophages sustains local and systemic interleukin-1β production in obesity. Sci Rep. 2020; 10:5555. PMID: 32221369.
Article13. Wiesner P, Choi SH, Almazan F, et al. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res. 2010; 107:56–65. PMID: 20489162.14. Deotare U, Al-Dawsari G, Couban S, Lipton JH. G-CSF-primed bone marrow as a source of stem cells for allografting: revisiting the concept. Bone Marrow Transplant. 2015; 50:1150–1156. PMID: 25915812.
Article15. Hathaway CK, Grant R, Hagaman JR, et al. Endothelin-1 critically influences cardiac function via superoxide-MMP9 cascade. Proc Natl Acad Sci U S A. 2015; 112:5141–5146. PMID: 25848038.
Article16. Tolar P, Wack A. Monocytes work harder under pressure. Nat Immunol. 2019; 20:1422–1424. PMID: 31595058.
Article17. Palomer X, Pizarro-Delgado J, Barroso E, Vázquez-Carrera M. Palmitic and oleic acid: the yin and yang of fatty acids in type 2 diabetes mellitus. Trends Endocrinol Metab. 2018; 29:178–190. PMID: 29290500.
Article18. Miller YI, Shyy JY. Context-dependent role of oxidized lipids and lipoproteins in inflammation. Trends Endocrinol Metab. 2017; 28:143–152. PMID: 27931771.
Article19. Grebe A, Hoss F, Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018; 122:1722–1740. PMID: 29880500.
Article20. Rhoads JP, Lukens JR, Wilhelm AJ, et al. Oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcγR cooperation and is dependent on CARD9. J Immunol. 2017; 198:2105–2114. PMID: 28130494.
Article21. Houben T, Brandsma E, Walenbergh SM, Hofker MH, Shiri-Sverdlov R. Oxidized LDL at the crossroads of immunity in non-alcoholic steatohepatitis. Biochim Biophys Acta Mol Cell Biol Lipids. 2017; 1862:416–429. PMID: 27472963.
Article22. Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019; 68:915–932. PMID: 31363792.
Article23. Miller YI, Choi SH, Wiesner P, Bae YS. The SYK side of TLR4: signalling mechanisms in response to LPS and minimally oxidized LDL. Br J Pharmacol. 2012; 167:990–999. PMID: 22776094.
Article24. Choi SH, Sviridov D, Miller YI. Oxidized cholesteryl esters and inflammation. Biochim Biophys Acta Mol Cell Biol Lipids. 2017; 1862:393–397. PMID: 27368140.
Article25. Camarena V, Sant D, Mohseni M, et al. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr Metab Cardiovasc Dis. 2017; 27:739–750. PMID: 28739185.
Article26. Karagiannis GS, Weile J, Bader GD, Minta J. Integrative pathway dissection of molecular mechanisms of moxLDL-induced vascular smooth muscle phenotype transformation. BMC Cardiovasc Disord. 2013; 13:4. PMID: 23324130.
Article27. Adlam D, Olson TM, Combaret N, et al. Association of the PHACTR1/EDN1 genetic locus with spontaneous coronary artery dissection. J Am Coll Cardiol. 2019; 73:58–66. PMID: 30621952.28. Denisenko E, Guler R, Mhlanga M, Suzuki H, Brombacher F, Schmeier S. Transcriptionally induced enhancers in the macrophage immune response to Mycobacterium tuberculosis infection. BMC Genomics. 2019; 20:71. PMID: 30669987.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diabetes and Dietary Fats
- Evaluation of Total Fat and Fatty Acids Intakes in the Korean Adult Population using Data from the 2016–2017 Korea National Health and Nutrition Examination Surveys
- High glucose, unsaturated and saturated fatty acids differentially regulate expression of ATP-binding cassette transporters ABCA1 and ABCG1 in human macrophages
- Regulating Hypothalamus Gene Expression in Food Intake: Dietary Composition or Calorie Density?
- Nonalcoholic Fatty Liver Disease: Lifestyle Modification